Mobility and transformation of mercury in the sediments of Changjiang estuarine wetlands following the soluble ionic mercury inputs:A long-term microcosm study
-
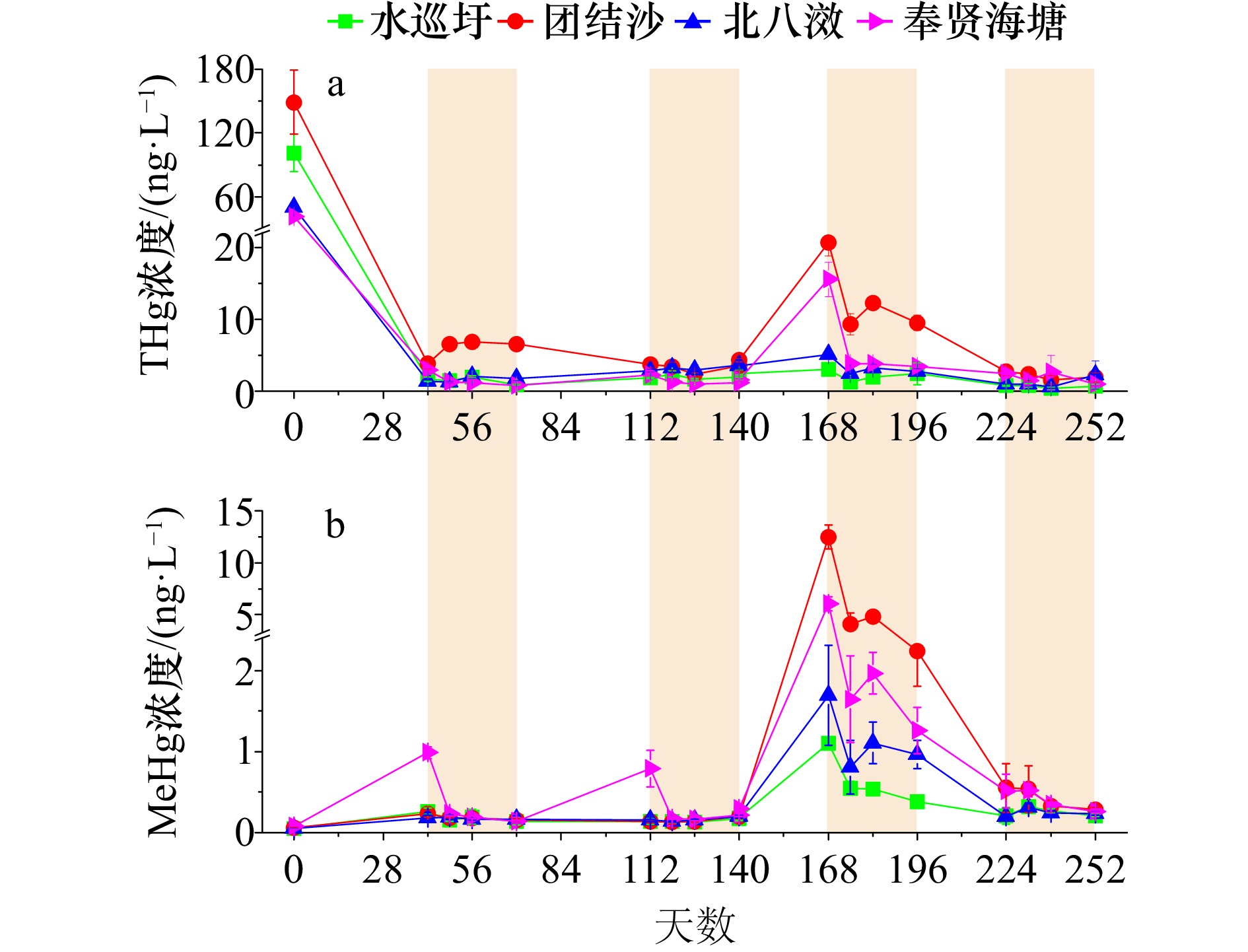
摘要: 利用微宇宙模拟试验探究了外源输入汞在长江口湿地沉积物氧化还原条件长时间持续变化过程中的迁移转化规律及其影响因素。添加溶解态硝酸汞模拟外源活性汞输入,4个沉积物总汞(THg)浓度增加109.7%~275.1%。252天培养试验结果显示:(1)溶解态活性汞输入沉积物后在亚还原及还原条件下易于转化为甲基汞(MeHg),与对照组相比,添加汞组MeHg浓度增加1.9%~657.3%(平均183.0%),尤其在培养140天后植物凋落物输入情境下,沉积物中MeHg平均增加260.2%,表明了易降解有机质输入及其腐解对活性汞老化的具有重要影响。同一采样点时间,各沉积物中汞甲基化潜势(MeHg/THg,%)显著不同,且均在凋落物厌氧腐解阶段显著升高,这可能是不同沉积物自身汞甲基化微生物差异所致。(2)沉积物氧化阶段,MeHg浓度与氧化还原电位值存在显著负相关关系,表明了再悬浮氧化过程会导致甲基汞的降解,且易降解有机质存在条件下甲基汞的降解作用增强,这可能是好氧微生物降解甲基汞与Fe(Ⅱ)氧化产生的活性氧物质化学降解甲基汞两种途径共同作用的结果,后者在河口海岸及其他水环境中的作用机理有待深入研究。(3)活性汞输入后主要累积于粒径<8 μm极细颗粒物中,甲基汞亦是如此,可能缘于黏土矿物-铁氧化物-有机质复合体对汞的吸附作用,表明了长江口极细颗粒物是汞迁移的重要载体。Abstract: To investigate the mobility and transformation of mercury (Hg) in the wetlands of Changjiang Estuary, microcosm incubation experiments were conducted under different redox conditions over a long period (252 days). Four sediments collected from different wetlands were added with dissolved Hg(NO3)2 to simulate recent Hg inputs to wetlands, resulting in sediment total Hg increased by 109.7−275.1%. (1) The results showed that the concentrations of methylmercury (MeHg) in sediments increased by 1.9−361.5% (on average 183.0%) over the course of incubation. Amendment of litterfall after 140 days incubation, anaerobic degradation of litter can significantly enhance MeHg production with a larger increase (on average 260.2%) compared to those in the control. These results suggest that soluble Hg is easily methylated to MeHg, especially with labile organic matter inputs, and the aging processes of Hg could be significantly influenced by labile organic matter. Furthermore, MeHg/THg (%), as an estimate of long-term MeHg production were significantly different among sediments for all sampling time points, which was most probably due to the differences of the Hg methylating bacteria in sediments. (2) During oxidation stage of the sediments, a significant negative correlation between the MeHg concentrations and the redox potential (Eh) was observed. The results indicate that MeHg demethylation occurred under oxic resuspension conditions, which was enhanced in the presence of labile organic matter. This was most probably due to the combination of biotic demethylation with aerobic microorganisms and abiotic demethylation associated with reactive oxygen species from oxygenation of Fe(II)-bearing particles. The role of the abiotic pathways and mechanisms in the degradation of methylmercury in estuaries, coasts and other natural aquatic systems needs to be further investigated. (3) Most of the Hg accumulated in the <8 μm fractions, probably due to the formation of Hg-organic matter complexes that was aggregated with metal (oxyhydr)oxides and clay minerals. Thus, very fine particles may be the main carriers of Hg in Changjiang Estuary.
-
Key words:
- Changjiang estuary /
- wetland /
- methylmercury /
- organic matter /
- sediment resuspension
-
图 7 培养期间泥浆氧化过程中沉积物汞甲基化浓度与溶液中Eh拟合关系(a);沉积物中背景汞甲基化潜势与净增加活性汞的甲基化潜势拟合关系(b)
(图a中三角表示未添加汞组(CK组和+OM组)氧化阶段样品,空心圆点表示添加汞组(+Hg组和+OM+Hg组)氧化阶段样品。图b中三角表示未添加植物凋落物组(CK组和+Hg组)样品,空心圆点表示添加植物凋落物组(+OM组和+OM+Hg组)样品)
Fig. 7 Relationship between the concentrations of MeHg in sediments and redox potential (Eh) in solutions during the oxidized stages of the incubation (a) and relationship between values of MeHg/THg (%) and ΔMeHg/ΔTHg (%) (b) over the course of sediment incubation
(In Fig. 7a, the triangles indicate samples from the un-amended groups (CK and +OM), and the circles indicate samples from the amended groups (+Hg and +OM+Hg) during the oxidation phase of incubation. In Fig. 7b, the triangles indicate the samples of groups without litter addition (CK and +Hg), and the circles indicate the samples of groups with litter addition (+OM and +OM+Hg) over the course of incubation)
表 1 供试沉积物理化参数(n = 3)
Tab. 1 Physicochemical parameters of the sediments used in our microcosm incubation experiments (n = 3)
处理组 参数 水巡圩 团结沙 北八滧 奉贤海塘 培养及取样时间 未添加汞处理组:对照组(CK)
和植物凋落物处理组(+OM)粒度D90/µm 33.0 36.2 25.0 22.7 CK组和+Hg组:6周密闭和4周氧化,第0、42、49、56、70、112、119、126、140天采样; TOC/% 0.9 ± 0.2 0.5 ± 0.3 1.3 ± 0.1 1.4 ± 0.0 总活性铁/(g·kg−1) 4.6 ± 0.1 4.1 ± 0.1 5.5 ± 0.1 5.5 ± 0.1 THg /(µg·kg−1) 56.6 ± 3.2 46.5 ± 5.7 78.7 ± 5.3 55.7 ± 4.2 MeHg /(µg·kg−1) 0.2 ± 0.0 0.3 ± 0.0 0.1 ± 0.0 0.5 ± 0.0 添加汞处理组:汞处理组(+Hg)
和汞与植物凋落物处理组(+OM+Hg)THg /(µg·kg−1) 136.1 ± 8.9 174.6 ± 6.5 165.0 ± 4.6 126.0 ± 9.5 +OM组和+OM+Hg组:4周密闭和4周氧化,第140、168、175、182、196、224、231、238、
252天采样THg相对增加/% 140.4 275.1 109.7 126.1 -
[1] United Nations Environment Programme (UNEP). Global mercury assessment 2018[EB/OL]. https://www.unep.org/resources/publication/global-mercury-assessment-2018, 2019-08-21. [2] Obrist D, Kirk J L, Zhang Lei, et al. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use[J]. Ambio, 2018, 47(2): 116−140. doi: 10.1007/s13280-017-1004-9 [3] Barbier E B, Hacker S D, Kennedy C, et al. The value of estuarine and coastal ecosystem services[J]. Ecological Monographs, 2011, 81(2): 169−193. doi: 10.1890/10-1510.1 [4] Krabbenhoft D P, Sunderland E M. Global change and mercury[J]. Science, 2013, 341(6153): 1457−1458. doi: 10.1126/science.1242838 [5] Hsu-Kim H, Eckley C S, Achá D, et al. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes[J]. Ambio, 2018, 47(2): 141−169. doi: 10.1007/s13280-017-1006-7 [6] Liu Maodian, Zhang Qianru, Yu Chenghao, et al. Observation-based mercury export from rivers to coastal oceans in East Asia[J]. Environmental Science & Technology, 2021, 55(20): 14269−14280. [7] Amos H M, Jacob D J, Kocman D, et al. Global biogeochemical implications of mercury discharges from rivers and sediment burial[J]. Environmental Science & Technology, 2014, 48(16): 9514−9522. [8] Hsu-Kim H, Kucharzyk K H, Zhang Tong, et al. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review[J]. Environmental Science & Technology, 2013, 47(6): 2441−2456. [9] Li Yanbin, Cai Yong. Progress in the study of mercury methylation and demethylation in aquatic environments[J]. Chinese Science Bulletin, 2013, 58(2): 177−185. doi: 10.1007/s11434-012-5416-4 [10] Liu Maodian, Mason R P, Vlahos P, et al. Riverine discharge fuels the production of methylmercury in a large temperate estuary[J]. Environmental Science & Technology, 2023, 57(35): 13056−13066. [11] Stein E D, Cohen Y, Winer A M. Environmental distribution and transformation of mercury compounds[J]. Critical Reviews in Environmental Science and Technology, 1996, 26(1): 1−43. doi: 10.1080/10643389609388485 [12] Mason R P, Choi A L, Fitzgerald W F, et al. Mercury biogeochemical cycling in the ocean and policy implications[J]. Environmental Research, 2012, 119: 101−117. doi: 10.1016/j.envres.2012.03.013 [13] Jonsson S, Skyllberg U, Nilsson M B, et al. Mercury methylation rates for geochemically relevant HgII species in sediments[J]. Environmental Science & Technology, 2012, 46(21): 11653−11659. [14] Liem-Nguyen V, Jonsson S, Skyllberg U, et al. Effects of nutrient loading and mercury chemical speciation on the formation and degradation of methylmercury in estuarine sediment[J]. Environmental Science & Technology, 2016, 50(13): 6983−6990. [15] Duan Dandan, Lei Pei, Lan Wenlu, et al. Litterfall-derived organic matter enhances mercury methylation in mangrove sediments of south China[J]. Science of the Total Environment, 2021, 765: 142763. doi: 10.1016/j.scitotenv.2020.142763 [16] Wang Yongjie, Wang Zhigang, Zheng Xiangmin, et al. Influence of Spartina alterniflora invasion on mercury storage and methylation in the sediments of Yangtze River estuarine wetlands[J]. Estuarine, Coastal and Shelf Science, 2022, 265: 107717. doi: 10.1016/j.ecss.2021.107717 [17] Zhu Wei, Song Yu, Adediran G A, et al. Mercury transformations in resuspended contaminated sediment controlled by redox conditions, chemical speciation and sources of organic matter[J]. Geochimica et Cosmochimica Acta, 2018, 220: 158−179. doi: 10.1016/j.gca.2017.09.045 [18] Sharif A, Monperrus M, Tessier E, et al. Fate of mercury species in the coastal plume of the Adour River estuary (bay of Biscay, SW France)[J]. Science of the Total Environment, 2014, 496: 701−713. doi: 10.1016/j.scitotenv.2014.06.116 [19] Seelen E A, Massey G M, Mason R P. Role of sediment resuspension on estuarine suspended particulate mercury dynamics[J]. Environmental Science & Technology, 2018, 52(14): 7736−7744. [20] Martı́n-Doimeadios R C R, Tessier E, Amouroux D, et al. Mercury methylation/demethylation and volatilization pathways in estuarine sediment slurries using species-specific enriched stable isotopes[J]. Marine Chemistry, 2004, 90(1/4): 107−123. [21] Barkay T, Gu Baohua. Demethylation-the other side of the mercury methylation coin: a critical review[J]. ACS Environmental Au, 2022, 2(2): 77−97. doi: 10.1021/acsenvironau.1c00022 [22] Yang Qingqing, Guo Yingying, Xiang Yuping, et al. Toward efficient bioremediation of methylmercury in sediment using merB overexpressed Escherichia coli[J]. Water Research, 2023, 229: 119502. doi: 10.1016/j.watres.2022.119502 [23] Baptista-Salazar C, Liem-Nguyen V, Jonsson S. Experiments revealing the formation of refractory methylmercury pools in natural sediments and soils[J]. Geochimica et Cosmochimica Acta, 2022, 328: 76−84. doi: 10.1016/j.gca.2022.04.009 [24] Liu Maodian, Zhang Wei, Wang Xuejun, et al. Mercury release to aquatic environments from anthropogenic sources in China from 2001 to 2012[J]. Environmental Science & Technology, 2016, 50(15): 8169−8177. [25] Liu Maodian, Xie Han, He Yipeng, et al. Sources and transport of methylmercury in the Yangtze River and the impact of the Three Gorges Dam[J]. Water Research, 2019, 166: 115042. doi: 10.1016/j.watres.2019.115042 [26] Cao Feng, Yang Shouye, Yin Daqiang, et al. Geochemical controls on the distribution of total mercury and methylmercury in sediments and porewater from the Yangtze River estuary to the East China Sea[J]. Science of the Total Environment, 2023, 892: 164737. doi: 10.1016/j.scitotenv.2023.164737 [27] 沈焕庭, 潘定安. 长江河口最大浑浊带[M]. 北京: 海洋出版社, 2001: 1−50.Shen Huanting, Pan Ding'an. Turbidity Maximum in the Changjiang Estuary[M]. Beijing: China Ocean Press, 2001: 1−50. (查阅网上资料, 本条文献的出版者的英文信息未找到, 请确认) [28] Yang Shilun, Li H, Ysebaert T, et al. Spatial and temporal variations in sediment grain size in tidal wetlands, Yangtze delta: on the role of physical and biotic controls[J]. Estuarine, Coastal and Shelf Science, 2008, 77(4): 657−671. doi: 10.1016/j.ecss.2007.10.024 [29] Olund S D, Dewild J F, Olson M L, et al. Methods for the Preparation and Analysis of Solids and Suspended Solids for Total Mercury[M]//Techniques and Methods 5: Laboratory Analysis, Section A: Water Analysis. Reston: United States Geological Survey, 2004, 8: 15. [30] Munson K M, Babi D, Lamborg C H. Determination of monomethylmercury from seawater with ascorbic acid-assisted direct ethylation[J]. Limnology and Oceanography: Methods, 2014, 12(1): 1−9. doi: 10.4319/lom.2014.12.1 [31] Liang Lian, Horvat M, Feng Xinbin, et al. Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples[J]. Applied Organometallic Chemistry, 2004, 18(6): 264−270. doi: 10.1002/aoc.617 [32] Dewild J F, Olund S D, Olson M L, et al. Methods for the Preparation and Analysis of Sofids and Suspended Solids for Methylmercury[M]//Techniques and Methods 5: Laboratory Analysis, Section A: Water Analysis. Reston: United States Geological Survey, 2004, 7: 1-12. [33] 王志刚, 周立旻, 郑祥民, 等. 长江河口湿地互花米草入侵对沉积物中汞形态特征的影响研究[J]. 海洋学报, 2021, 43(8): 31−40.Wang Zhigang, Zhou Limin, Zheng Xiangmin, et al. Effects of Spartina alterniflora invasion on mercury speciation in vegetated sediments of the wetland in Changjiang River estuary, China[J]. Haiyang Xuebao, 2021, 43(8): 31−40. [34] Zhao Guoqiang, Wu Binbin, Zheng Xiaoshan, et al. Tide-triggered production of reactive oxygen species in coastal soils[J]. Environmental Science & Technology, 2022, 56(16): 11888−11896. [35] Drott A, Lambertsson L, Björn E, et al. Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments?[J]. Environmental Science & Technology, 2008, 42(1): 153−158. [36] Ullrich S M, Tanton T W, Abdrashitova S A. Mercury in the aquatic environment: a review of factors affecting methylation[J]. Critical Reviews in Environmental Science and Technology, 2001, 31(3): 241−293. doi: 10.1080/20016491089226 [37] Jonsson S, Skyllberg U, Nilsson M B, et al. Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota[J]. Nature Communications, 2014, 5(1): 4624. doi: 10.1038/ncomms5624 [38] Wadle A, Neal-Walthall N, Ndu U, et al. Distribution and homogenization of multiple mercury species inputs to freshwater wetland mesocosms[J]. Environmental Science & Technology, 2024, 58(3): 1709−1720. [39] Zhu Changle, Lv Shaoyang, Zhao Qing, et al. Seasonal variation in mercury and methylmercury production in vegetated sediment in the Dongtan wetlands of the Yangtze River estuary, China[J]. Marine Environmental Research, 2023, 188: 105999. doi: 10.1016/j.marenvres.2023.105999 [40] Bravo A G, Bouchet S, Tolu J, et al. Molecular composition of organic matter controls methylmercury formation in boreal lakes[J]. Nature Communications, 2017, 8(1): 14255. doi: 10.1038/ncomms14255 [41] Lin Heyu, Ascher D B, Myung Y, et al. Mercury methylation by metabolically versatile and cosmopolitan marine bacteria[J]. The ISME Journal, 2021, 15(6): 1810−1825. doi: 10.1038/s41396-020-00889-4 [42] Jiang Tao, Bravo A G, Skyllberg U, et al. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China[J]. Water Research, 2018, 146: 146−158. doi: 10.1016/j.watres.2018.08.054 [43] Xie Fuyu, Yuan Qingke, Meng Ying, et al. Degradation of methylmercury into Hg(0) by the oxidation of iron(II) minerals[J]. Water Research, 2024, 256: 121645. doi: 10.1016/j.watres.2024.121645 [44] Yu Chenglong, Lu Yuxi, Zhang Yanting, et al. Significant contribution of solid organic matter for hydroxyl radical production during oxygenation[J]. Environmental Science & Technology, 2022, 56(16): 11878−11887. [45] Wu Binbin, Zhou Chong, Zhao Guoqiang, et al. Enhanced photochemical production of reactive intermediates at the wetland soil-water interface[J]. Water Research, 2022, 223: 118971. doi: 10.1016/j.watres.2022.118971 [46] Turner A, Millward G E, Le Roux S M. Significance of oxides and particulate organic matter in controlling trace metal partitioning in a contaminated estuary[J]. Marine Chemistry, 2004, 88(3/4): 179−192. [47] Gu B, Mishra B, Miller C, et al. X-ray fluorescence mapping of mercury on suspended mineral particles and diatoms in a contaminated freshwater system[J]. Biogeosciences, 2014, 11(18): 5259−5267. doi: 10.5194/bg-11-5259-2014 [48] 李道季, 李军, 陈吉余, 等. 长江河口悬浮颗粒物研究[J]. 海洋与湖沼, 2000, 31(3): 295−301. doi: 10.3321/j.issn:0029-814X.2000.03.010Li Daoji, Li Jun, Chen Jiyu, et al. A study on suspended matter in the Changjiang River estuary[J]. Oceanologia et Limnologia Sinica, 2000, 31(3): 295−301. doi: 10.3321/j.issn:0029-814X.2000.03.010 -




 下载:
下载: