Progress and prospect of bacterial membrane vesicles in marine ecosystem
-
摘要: 膜囊泡是一类由细菌释放并携带复杂蛋白质、核酸、信号分子等重要信息物质的生物纳米颗粒,参与了水平基因转移、群体感应、生物膜形成等多种生理生化活动。海洋中的细胞膜囊泡可能是除噬菌体和细菌之外的第三大生物实体,其介导的跨越物种的细胞间通讯对海洋生态系统而言有重要意义。然而,我们对膜囊泡在海洋生物圈中具体的生态角色与生物功能所知甚少。因此,本综述从细菌膜囊泡在海洋微生态、海洋共生体系中的作用及其物质流转对生态系统的影响等方面开展讨论,分析了目前细菌膜囊泡在海洋生态功能研究中尚未解决的科学问题,并对未来的研究方向进行了展望。Abstract: Bacterial membrane vesicles (MVs), which are a kind of biological nanoparticles carrying proteins, nucleic acids, signaling molecules, and other important compounds, are involved in a variety of physiological and biochemical processes, such as horizontal gene transfer, quorum sensing, biofilm formation and so on. Studies showed that marine extracellular vesicles might be the third largest biological entity except for phages and bacteria. Besides, MVs-mediated intercellular communication across species could be of great significance to marine ecosystems. However, very little is known about the specific ecological role and biological function of MVs in the marine biosphere. In this review, we discuss the role of bacterial MVs in marine micro-ecology, marine symbiotic system, and the impact of MVs-mediated material delivery on marine ecosystem. Meanwhile, we also put forward some questions and opinions for study in marine bacterial MVs’ research.
-
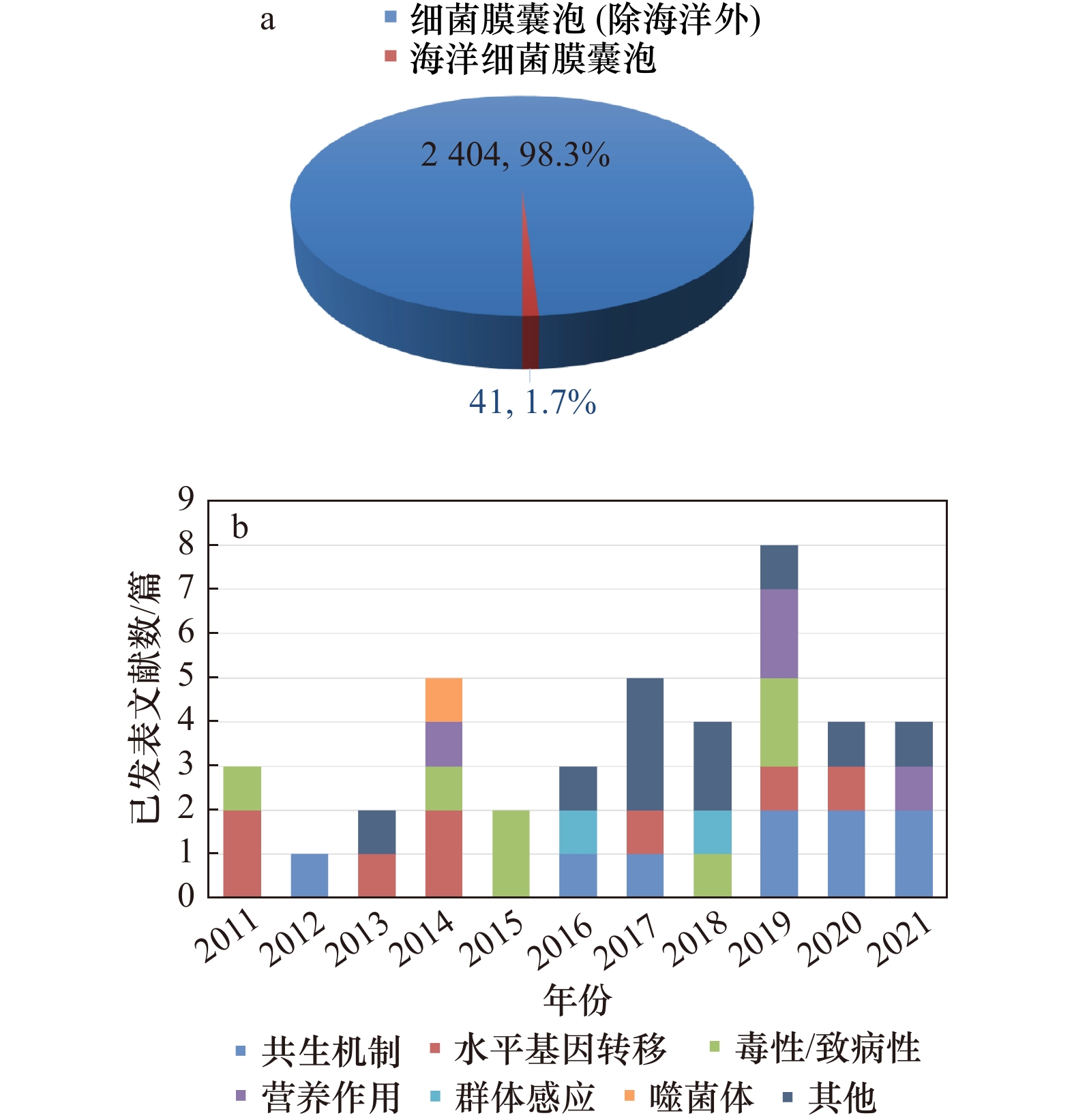
图 2 2011−2021年发表的海洋细菌膜囊泡领域文献数量
a. 海洋领域膜囊泡文献数量占细菌膜囊泡研究的1.7%;b. 海洋细菌膜囊泡领域的不同研究方向
Fig. 2 Publications in the field of marine bacterial membrane vesicles from 2011 to 2021
a. The number of publications in the field of marine membrane vesicles accounts for 1.7% of the study in bacterial membrane vesicles; b. different research directions in the field of marine bacterial membrane vesicles
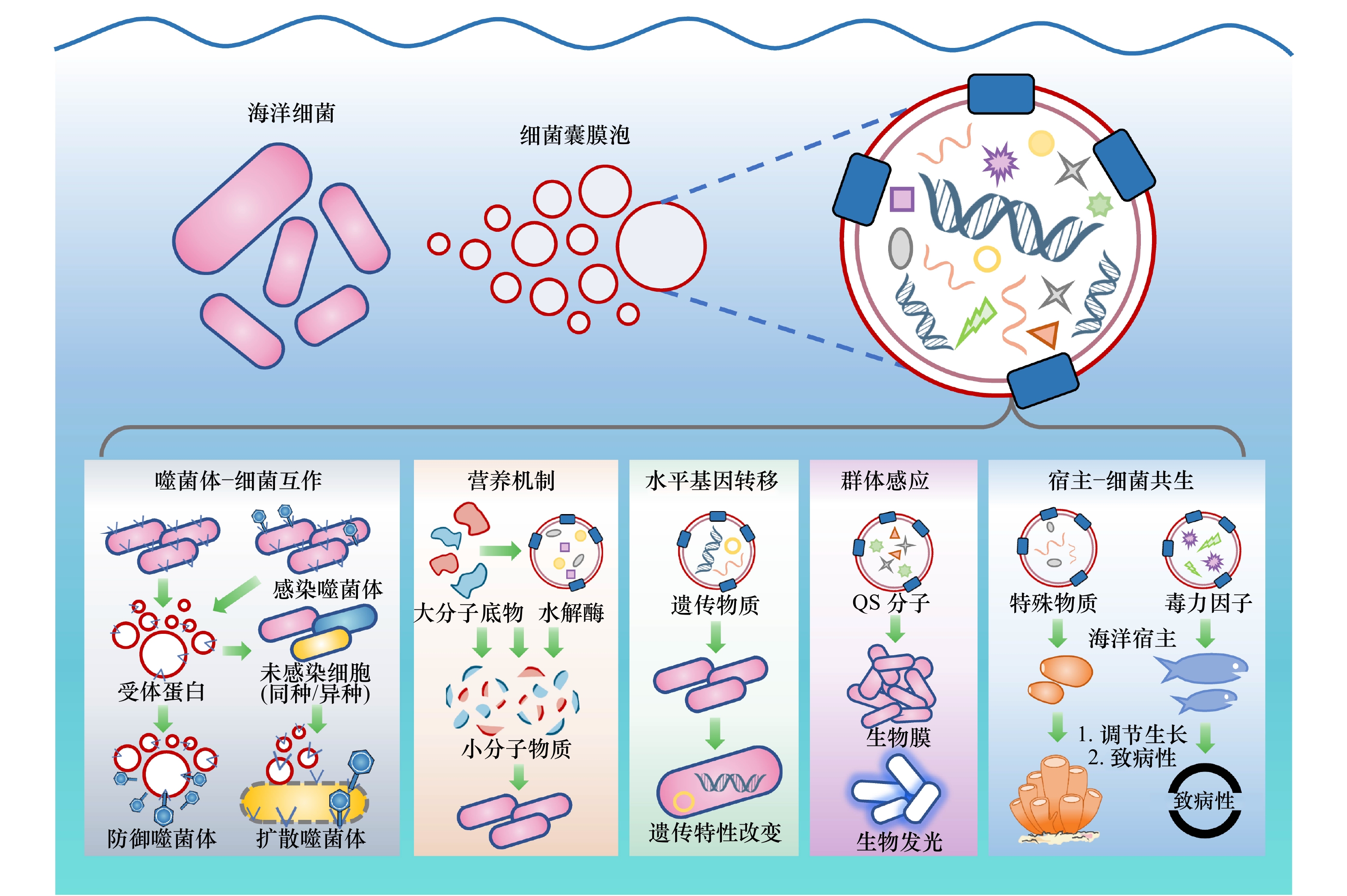
图 4 细菌膜囊泡参与噬菌体与微生物相互作用
a. 细菌膜囊泡作为诱饵诱骗噬菌体;b. 细菌膜囊泡介导细胞间物质交换,促进噬菌体感染
Fig. 4 Bacterial membrane vesicles are involved in phage-bacterial interactions
a. Bacterial membrane vesicles function as decoys for phages; b. bacterial membrane vesicles transfer material between cells and facilitate phage infection
-
[1] Darwin C R. The Variation of Animals and Plants under Domestication[M]. Landon: John Murray, 1868. [2] Chatterjee S N, Das J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae[J]. Journal of General Microbiology, 1967, 49(1): 1−11. doi: 10.1099/00221287-49-1-1 [3] Knox K W, Vesk M, Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli[J]. Journal of Bacteriology, 1966, 92(4): 1206−1217. doi: 10.1128/jb.92.4.1206-1217.1966 [4] DeVoe I W, Gilchrist J E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease[J]. Journal of Experimental Medicine, 1975, 141(2): 297−305. doi: 10.1084/jem.141.2.297 [5] Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nature Cell Biology, 2007, 9(6): 654−659. doi: 10.1038/ncb1596 [6] Kalluri R, LeBleu V S. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. doi: 10.1126/science.aau6977 [7] Roier S, Zingl F G, Cakar F, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria[J]. Nature Communications, 2016, 7: 10515. doi: 10.1038/ncomms10515 [8] Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles[J]. Nature Reviews Microbiology, 2019, 17(1): 13−24. doi: 10.1038/s41579-018-0112-2 [9] Furuyama N, Sircili M P. Outer membrane vesicles (OMVs) produced by Gram-negative bacteria: structure, functions, biogenesis, and vaccine application[J]. BioMed Research International, 2021, 2021: 1490732. [10] Florez C, Raab J E, Cooke A C, et al. Membrane distribution of the Pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa[J]. mBio, 2017, 8(4): e01034−17. [11] Schwechheimer C, Kulp A, Kuehn M J. Modulation of bacterial outer membrane vesicle production by envelope structure and content[J]. BMC Microbiology, 2014, 14: 324. doi: 10.1186/s12866-014-0324-1 [12] Tashiro Y, Sakai R, Toyofuku M, et al. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa[J]. Journal of Bacteriology, 2009, 191(24): 7509−7519. doi: 10.1128/JB.00722-09 [13] Schertzer J W, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis[J]. mBio, 2012, 3(2): e00297−11. [14] Hayashi J, Hamada N, Kuramitsu H K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release[J]. FEMS Microbiology Letters, 2002, 216(2): 217−222. doi: 10.1111/j.1574-6968.2002.tb11438.x [15] Turnbull L, Toyofuku M, Hynen A L, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms[J]. Nature Communications, 2016, 7: 11220. doi: 10.1038/ncomms11220 [16] Brown L, Wolf J M, Prados-Rosales R, et al. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi[J]. Nature Reviews Microbiology, 2015, 13(10): 620−630. doi: 10.1038/nrmicro3480 [17] Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis[J]. Nature Communications, 2017, 8(1): 481. doi: 10.1038/s41467-017-00492-w [18] Altindis E, Fu Yang, Mekalanos J J. Proteomic analysis of Vibrio cholerae outer membrane vesicles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(15): E1548−E1556. [19] Zingl F G, Thapa H B, Scharf M, et al. Outer membrane vesicles of Vibrio cholerae protect and deliver active cholera toxin to host cells via porin-dependent uptake[J]. mBio, 2021, 12(3): e00534−21. [20] Lin Jinshui, Zhang Weipeng, Cheng Juanli, et al. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition[J]. Nature Communications, 2017, 8: 14888. doi: 10.1038/ncomms14888 [21] Prados-Rosales R, Weinrick B C, Piqué D G, et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition[J]. Journal of Bacteriology, 2014, 196(6): 1250−1256. doi: 10.1128/JB.01090-13 [22] Tran F, Boedicker J Q. Plasmid characteristics modulate the propensity of gene exchange in bacterial vesicles[J]. Journal of Bacteriology, 2019, 201(7): e00430−18. [23] Yaron S M, Kolling G L, Simon L, et al. Vesicle-mediated transfer of virulence genes from Escherichia coli O157: H7 to other enteric bacteria[J]. Applied and Environmental Microbiology, 2000, 66(10): 4414−4420. doi: 10.1128/AEM.66.10.4414-4420.2000 [24] Schaefer A L, Taylor T A, Beatty J T, et al. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production[J]. Journal of Bacteriology, 2002, 184(23): 6515−6521. doi: 10.1128/JB.184.23.6515-6521.2002 [25] Bielig H, Dongre M, Zurek B, et al. A role for quorum sensing in regulating innate immune responses mediated by Vibrio cholerae outer membrane vesicles (OMVs)[J]. Gut Microbes, 2011, 2(5): 274−279. doi: 10.4161/gmic.2.5.18091 [26] Schooling S R, Hubley A, Beveridge T J. Interactions of DNA with biofilm-derived membrane vesicles[J]. Journal of Bacteriology, 2009, 191(13): 4097−4102. doi: 10.1128/JB.00717-08 [27] Yonezawa H, Osaki T, Kurata S, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation[J]. BMC Microbiology, 2009, 9: 197. doi: 10.1186/1471-2180-9-197 [28] Biller S J, Schubotz F, Roggensack S E, et al. Bacterial vesicles in marine ecosystems[J]. Science, 2014, 343(6167): 183−186. doi: 10.1126/science.1243457 [29] Manning A J, Kuehn M J. Contribution of bacterial outer membrane vesicles to innate bacterial defense[J]. BMC Microbiology, 2011, 11: 258. doi: 10.1186/1471-2180-11-258 [30] Li Jie, Azam F, Zhang Si. Outer membrane vesicles containing signalling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1[J]. Environmental Microbiology, 2016, 18(11): 3850−3866. doi: 10.1111/1462-2920.13344 [31] MacDonald I A, Kuehn M J. Offense and defense: microbial membrane vesicles play both ways[J]. Research in Microbiology, 2012, 163(9/10): 607−618. [32] Schatz D, Rosenwasser S, Malitsky S, et al. Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga[J]. Nature Microbiology, 2017, 2(11): 1485−1492. doi: 10.1038/s41564-017-0024-3 [33] Alegado R A, Brown L W, Cao Shugeng, et al. A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals[J]. eLife, 2012, 1: e00013. doi: 10.7554/eLife.00013 [34] Woznica A, Cantley A M, Beemelmanns C, et al. Bacterial lipids activate, synergize, and inhibit a developmental switch in choanoflagellates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(28): 7894−7899. doi: 10.1073/pnas.1605015113 [35] Ireland E V, Woznica A, King N. Synergistic cues from diverse bacteria enhance multicellular development in a choanoflagellate[J]. Applied and Environmental Microbiology, 2020, 86(11): e02920−19. [36] Li Mingyu, Wang Kai, Jia Chenzheng, et al. Bacteroidetes bacteria, important players in the marine sponge larval development process[J]. iScience, 2021, 24(6): 102662. doi: 10.1016/j.isci.2021.102662 [37] Guo Huijuan, Rischer M, Westermann M, et al. Two distinct bacterial biofilm components trigger metamorphosis in the colonial hydrozoan Hydractinia echinata[J]. mBio, 2021, 12(3): e00401−21. [38] Freckelton M L, Nedved B T, Hadfield M G. Induction of invertebrate larval settlement; different bacteria, different mechanisms?[J]. Scientific Reports, 2017, 7: 42557. doi: 10.1038/srep42557 [39] Lynch J B, Schwartzman J A, Bennett B D, et al. Ambient pH alters the protein content of outer membrane vesicles, driving host development in a beneficial symbiosis[J]. Journal of Bacteriology, 2019, 201(20): e00319−19. [40] Lynch J B, Alegado R A. Spheres of hope, packets of doom: the good and bad of outer membrane vesicles in interspecies and ecological dynamics[J]. Journal of Bacteriology, 2017, 199(15): e00012−17. [41] Brameyer S, Plener L, Müller A, et al. Outer membrane vesicles facilitate trafficking of the hydrophobic signaling molecule CAI-1 between Vibrio harveyi cells[J]. Journal of Bacteriology, 2018, 200(15): e00740−17. [42] Naval P, Chandra T S. Characterization of membrane vesicles secreted by seaweed associated bacterium Alteromonas macleodii KS62[J]. Biochemical and Biophysical Research Communications, 2019, 514(2): 422−427. doi: 10.1016/j.bbrc.2019.04.148 [43] Harvey H, Bondy-Denomy J, Marquis H, et al. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation[J]. Nature Microbiology, 2018, 3(1): 47−52. doi: 10.1038/s41564-017-0061-y [44] Seed K D, Yen M, Shapiro B J, et al. Evolutionary consequences of intra-patient phage predation on microbial populations[J]. eLife, 2014, 3: e03497. doi: 10.7554/eLife.03497 [45] Loenen W A M, Dryden D T F, Raleigh E A, et al. Highlights of the DNA cutters: a short history of the restriction enzymes[J]. Nucleic Acids Research, 2014, 42(1): 3−19. doi: 10.1093/nar/gkt990 [46] Barrangou R, Fremaux C, Deveau H, et al. CRISPR provides acquired resistance against viruses in prokaryotes[J]. Science, 2007, 315(5819): 1709−1712. doi: 10.1126/science.1138140 [47] Chopin M C, Chopin A, Bidnenko E. Phage abortive infection in lactococci: variations on a theme[J]. Current Opinion in Microbiology, 2005, 8(4): 473−479. doi: 10.1016/j.mib.2005.06.006 [48] Wang Shiwei, Wan Mengping, Huang Ruolin, et al. SspABCD-SspFGH constitutes a new type of DNA phosphorothioate-based bacterial defense system[J]. mBio, 2021, 12(2): e00613−21. [49] Reyes-Robles T, Dillard R S, Cairns L S, et al. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection[J]. Journal of Bacteriology, 2018, 200(15): e00792−17. [50] Suttle C A. Marine viruses—major players in the global ecosystem[J]. Nature Reviews Microbiology, 2007, 5(10): 801−812. doi: 10.1038/nrmicro1750 [51] Wommack K E, Colwell R R. Virioplankton: viruses in aquatic ecosystems[J]. Microbiology and Molecular Biology Reviews, 2000, 64(1): 69−114. doi: 10.1128/MMBR.64.1.69-114.2000 [52] Loeb M R, Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1978, 514(1): 117−127. doi: 10.1016/0005-2736(78)90081-0 [53] Gaudin M, Krupovic M, Marguet E, et al. Extracellular membrane vesicles harbouring viral genomes[J]. Environmental Microbiology, 2014, 16(4): 1167−1175. doi: 10.1111/1462-2920.12235 [54] Kharina A, Podolich O, Faidiuk I, et al. Temperate bacteriophages collected by outer membrane vesicles in Komagataeibacter intermedius[J]. Journal of Basic Microbiology, 2015, 55(4): 509−513. doi: 10.1002/jobm.201400711 [55] Tzipilevich E, Habusha M, Ben-Yehuda S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors[J]. Cell, 2017, 168(1/2): 186−199.e12. [56] Hassanpour M, Rezaie J, Nouri M, et al. The role of extracellular vesicles in COVID-19 virus infection[J]. Infection, Genetics and Evolution, 2020, 85: 104422. doi: 10.1016/j.meegid.2020.104422 [57] Konadu K A, Chu J, Huang M B, et al. Association of cytokines with exosomes in the plasma of HIV-1-seropositive individuals[J]. The Journal of Infectious Diseases, 2015, 211(11): 1712−1716. doi: 10.1093/infdis/jiu676 [58] Raab-Traub N, Dittmer D P. Viral effects on the content and function of extracellular vesicles[J]. Nature Reviews Microbiology, 2017, 15(9): 559−572. doi: 10.1038/nrmicro.2017.60 [59] Toledo A, Coleman J L, Kuhlow C J, et al. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles[J]. Infection and Immunity, 2012, 80(1): 359−368. doi: 10.1128/IAI.05836-11 [60] Evans A G L, Davey H M, Cookson A, et al. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo[J]. Microbiology, 2012, 158(11): 2742−2752. doi: 10.1099/mic.0.060343-0 [61] Vasilyeva N V, Tsfasman I M, Suzina N E, et al. Secretion of bacteriolytic endopeptidase l5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles[J]. The FEBS Journal, 2008, 275(15): 3827−3835. doi: 10.1111/j.1742-4658.2008.06530.x [62] Zengler K, Zaramela L S. The social network of microorganisms—how auxotrophies shape complex communities[J]. Nature Reviews Microbiology, 2018, 16(6): 383−390. doi: 10.1038/s41579-018-0004-5 [63] Schatz D, Vardi A. Extracellular vesicles—new players in cell-cell communication in aquatic environments[J]. Current Opinion in Microbiology, 2018, 43: 148−154. doi: 10.1016/j.mib.2018.01.014 [64] Zehr J P, Weitz J S, Joint I. How microbes survive in the open ocean[J]. Science, 2017, 357(6352): 646−647. doi: 10.1126/science.aan5764 [65] Dürwald A, Zühlke M K, Schlüter R, et al. Reaching out in anticipation: Bacterial membrane extensions represent a permanent investment in polysaccharide sensing and utilization[J]. Environmental Microbiology, 2021, 23(6): 3149−3163. doi: 10.1111/1462-2920.15537 [66] Fischer T, Schorb M, Reintjes G, et al. Biopearling of interconnected outer membrane vesicle chains by a marine flavobacterium[J]. Applied and Environmental Microbiology, 2019, 85(19): e00829−19. [67] Pérez-Cruz C, Carrión O, Delgado L, et al. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content[J]. Applied and Environmental Microbiology, 2013, 79(6): 1874−1881. doi: 10.1128/AEM.03657-12 [68] Nevot M, Deroncelé V, Messner P, et al. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3[J]. Environmental Microbiology, 2006, 8(9): 1523−1533. doi: 10.1111/j.1462-2920.2006.01043.x [69] Ireland M M E, Karty J A, Quardokus E M, et al. Proteomic analysis of the Caulobacter crescentus stalk indicates competence for nutrient uptake[J]. Molecular Microbiology, 2002, 45(4): 1029−1041. doi: 10.1046/j.1365-2958.2002.03071.x [70] Chiura H X, Kogure K, Hagemann S, et al. Evidence for particle-induced horizontal gene transfer and serial transduction between bacteria[J]. FEMS Microbiology Ecology, 2011, 76(3): 576−591. doi: 10.1111/j.1574-6941.2011.01077.x [71] Lengeler J W, Drews G, Schlegel H G. Biology of the Prokaryotes[M]. Stuttgart: Georg Thieme Verlag, 1998. [72] Dorward D W, Garon C F, Judd R C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae[J]. Journal of Bacteriology, 1989, 171(5): 2499−2505. doi: 10.1128/jb.171.5.2499-2505.1989 [73] Chatterjee S, Mondal A, Mitra S, et al. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles[J]. Journal of Antimicrobial Chemotherapy, 2017, 72(8): 2201−2207. doi: 10.1093/jac/dkx131 [74] Klieve A V, Yokoyama M T, Forster R J, et al. Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin[J]. Applied and Environmental Microbiology, 2005, 71(8): 4248−4253. doi: 10.1128/AEM.71.8.4248-4253.2005 [75] Domingues S, Nielsen K M. Membrane vesicles and horizontal gene transfer in prokaryotes[J]. Current Opinion in Microbiology, 2017, 38: 16−21. doi: 10.1016/j.mib.2017.03.012 [76] Biller S J, McDaniel L D, Breitbart M, et al. Membrane vesicles in sea water: heterogeneous DNA content and implications for viral abundance estimates[J]. The ISME Journal, 2017, 11(2): 394−404. doi: 10.1038/ismej.2016.134 [77] Soler N, Marguet E, Verbavatz J M, et al. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales[J]. Research in Microbiology, 2008, 159(5): 390−399. doi: 10.1016/j.resmic.2008.04.015 [78] Gaudin M, Gauliard E, Schouten S, et al. Hyperthermophilic archaea produce membrane vesicles that can transfer DNA[J]. Environmental Microbiology Reports, 2013, 5(1): 109−116. doi: 10.1111/j.1758-2229.2012.00348.x [79] Erdmann S, Tschitschko B, Zhong Ling, et al. A plasmid from an Antarctic haloarchaeon uses specialized membrane vesicles to disseminate and infect plasmid-free cells[J]. Nature Microbiology, 2017, 2(10): 1446−1455. doi: 10.1038/s41564-017-0009-2 [80] Kwon Y M, Patra A K, Chiura H X, et al. Production of extracellular vesicles with light-induced proton pump activity by proteorhodopsin-containing marine bacteria[J]. MicrobiologyOpen, 2019, 8(8): e00808. [81] Lee J, Zhang Lianhui. The hierarchy quorum sensing network in Pseudomonas aeruginosa[J]. Protein & Cell, 2015, 6(1): 26−41. [82] Drees B, Reiger M, Jung K, et al. A modular view of the diversity of cell-density-encoding schemes in bacterial quorum-sensing systems[J]. Biophysical Journal, 2014, 107(1): 266−277. doi: 10.1016/j.bpj.2014.05.031 [83] Mashburn L M, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote[J]. Nature, 2005, 437(7057): 422−425. doi: 10.1038/nature03925 [84] Toyofuku M, Morinaga K, Hashimoto Y, et al. Membrane vesicle-mediated bacterial communication[J]. The ISME Journal, 2017, 11(6): 1504−1509. doi: 10.1038/ismej.2017.13 [85] Chung H, Pamp S J, Hill J A, et al. Gut immune maturation depends on colonization with a host-specific microbiota[J]. Cell, 2012, 149(7): 1578−1593. doi: 10.1016/j.cell.2012.04.037 [86] Ñahui Palomino R A, Vanpouille C, Laghi L, et al. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues[J]. Nature Communications, 2019, 10(1): 5656. doi: 10.1038/s41467-019-13468-9 [87] Chu H, Khosravi A, Kusumawardhani I P, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease[J]. Science, 2016, 352(6289): 1116−1120. doi: 10.1126/science.aad9948 [88] Deo P, Chow S H, Han Meiling, et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation[J]. Nature Microbiology, 2020, 5(11): 1418−1427. doi: 10.1038/s41564-020-0773-2 [89] Apprill A. The role of symbioses in the adaptation and stress responses of marine organisms[J]. Annual Review of Marine Science, 2020, 12: 291−314. doi: 10.1146/annurev-marine-010419-010641 [90] Laanto E, Penttinen R K, Bamford J K H, et al. Comparing the different morphotypes of a fish pathogen-implications for key virulence factors in Flavobacterium columnare[J]. BMC Microbiology, 2014, 14: 170. doi: 10.1186/1471-2180-14-170 [91] Wen Ying, Chen Shouwen, Jiang Zhiwei, et al. Dysregulated haemolysin promotes bacterial outer membrane vesicles-induced pyroptotic-like cell death in zebrafish[J]. Cellular Microbiology, 2019, 21(6): e13010. doi: 10.1111/cmi.13010 [92] Vanhove A S, Duperthuy M, Charrière G M, et al. Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells[J]. Environmental Microbiology, 2015, 17(4): 1152−1165. doi: 10.1111/1462-2920.12535 -




 下载:
下载: