Preliminary functional study of foxl2 in Portunus trituberculatus and analysis of its related miRNA
-
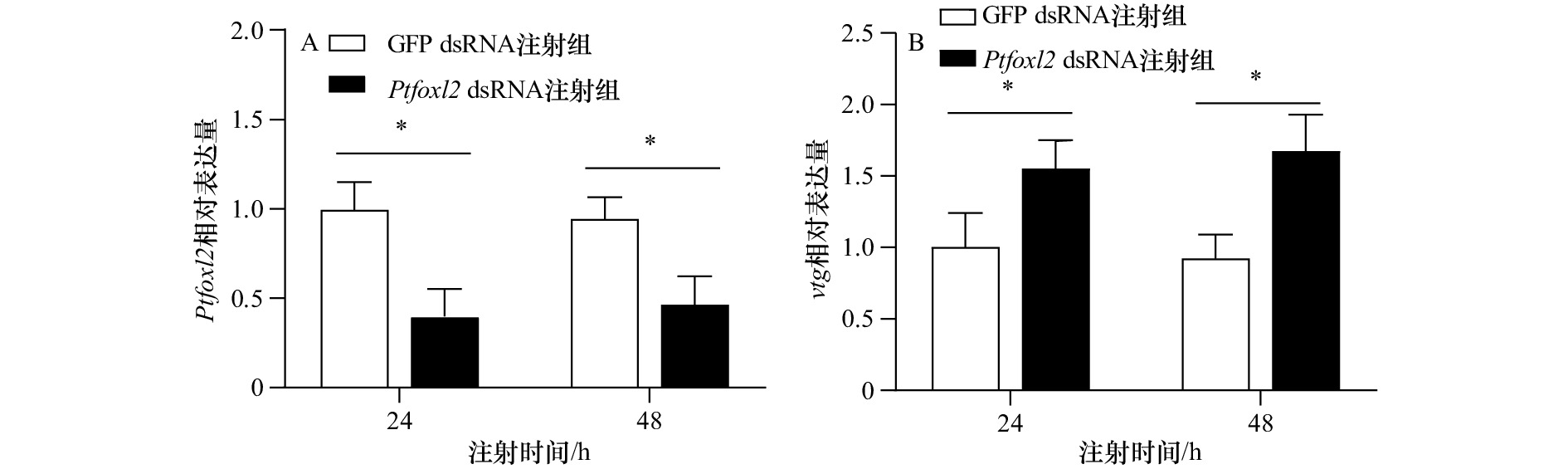
摘要: foxl2在脊椎动物卵巢分化、发育和功能维持等方面具有重要作用,然而其在三疣梭子蟹(Portunus trituberculatus)卵巢发育中的功能尚不明确。本研究首先克隆了三疣梭子蟹foxl2(Ptfoxl2)基因cDNA全长序列,该基因5′和3′非编码区域(UTR)长度分别为701 bp和211 bp,开放阅读框的长度为1 590 bp。基因表达分析结果显示,foxl2在三疣梭子蟹不同组织中均有表达,但在卵巢中表达量最高;其在卵巢发育不同时期的表达存在显著差异,在V期表达量最高;切除眼柄后,该基因的表达出现显著下降;干扰该基因表达后,卵巢vtg基因的表达显著上调。上述结果表明,foxl2可能在三疣梭子蟹卵巢发育调控中发挥重要功能,能够抑制卵巢组织中卵黄蛋白的合成。为进一步分析该基因的表达调控方式,利用生物信息学方法,预测了靶向foxl2的miRNA,并通过双荧光素酶报告基因检测实验,从细胞水平验证了这些miRNA对Ptfoxl2的调控作用;分析了其在卵巢发育不同时期以及切除眼柄后的表达模式。结果显示,共转染miR-9类似物和包含foxl2 3′UTR的pmirGLO质粒组,萤火虫酶与海肾荧光素酶活性比值出现显著下降,且在卵巢发育过程及切除眼柄后与foxl2表达模式相反。该结果初步证实miR-9可以从转录后水平调控三疣梭子蟹foxl2基因的表达。Abstract: The foxl2 plays essential roles in regulating ovarian differentiation, development and functional maintenance of vertebrate. However, its function in ovarian development of the swimming crab Portunus trituberculatus remains unknown. In the present study, we firstly cloned the full-length cDNA of P. trituberculatus foxl2 (Ptfoxl2) which contained a 5′ untranslated region (UTR) of 701 bp, a 3′ UTR of 211 bp, and an open reading frame (ORF) 1590 bp. Gene expression analysis in different tissues showed that Ptfoxl2 exhibited the highest expression in ovary. There were significant differences in its expression in different stages of ovarian development, and its expression was the highest in stage V. After eyestalk ablation, Ptfoxl2 expression decreased significantly. In addition, RNAi of Ptfoxl2 resulted in a significant reduction in vitellogenin expression of ovary. Taken together, these results indicated that Ptfoxl2 played an important role in the regulation of ovarian development of P. trituberculatus by inhibiting endogenous vitellogenesis in maturation stage. In order to further analyze the post-transcriptional regulation of Ptfoxl2, the miRNAs targeting Ptfoxl2 was predicted by bioinformatics analysis and the interaction between the miRNAs and Ptfoxl2 was verified using the dual luciferase reporter assay. The results showed that the relative luciferase activity was significantly reduced after co-transfection of miR-9 mimics and pmirGLO-Ptfoxl2-3′ UTR. Expression analysis showed that miR-9 exhibited opposite pattern at ovarian maturation stage and after eyestalk ablation. Collectively, the results demonstrated that miR-9 can regulate Ptfoxl2 expression in the swimming crab.
-
Key words:
- Portunus trituberculatus /
- foxl2 /
- eyestalk ablation /
- ovarian development /
- miRNA
-
图 1 Ptfoxl2基因cDNA全长序列以及推导的氨基酸序列
FH结构域用阴影表示;起始密码子和终止密码子用方框标出;下划线标注区域依次分别为miR-9、novel-68和novel-52的预测结合位点;左侧数字为核苷酸和氨基酸位置
Fig. 1 Nucleotide sequence and deduced amino acid sequence of Ptfoxl2 gene
FH domain was marked with shadow; initiation codon and stop codon were marked with black box; the underlined regions were the predicted binding sites for miR-9, novel-68, and novel-52 in turn; the numbers on the left indicate the positions of nucleotide and amino acid
图 2 4种甲壳动物foxl2氨基酸序列多重比对
右侧数字为氨基酸位置;各物种foxl2基因序列GenBank登录号:三疣梭子蟹(OK413951)、拟穴青蟹(MN412580.1)、中华绒螯蟹(KF806733.1)、斑节对虾(XM_037939236.1);黑色代表氨基酸残基同源性100%;红色代表氨基酸残基同源性75%;蓝色代表氨基酸残基同源性50%
Fig. 2 Multiple alignments of the amino acid sequences of foxl2 in four crustacean species
The numbers on the right indicate the positions of the amino acids; the GenBank accession numbers of foxl2 gene were as follows: Portunus trituberculatus (OK413951), Scylla paramamosain (MN412580.1), Eriocheir sinensis (KF806733.1), Penaeus monodon (XM_037939236.1); black represents 100% homology of amino acid residues; red represents 75% homology of amino acid residues; blue represents 50% homology of amino acid residues
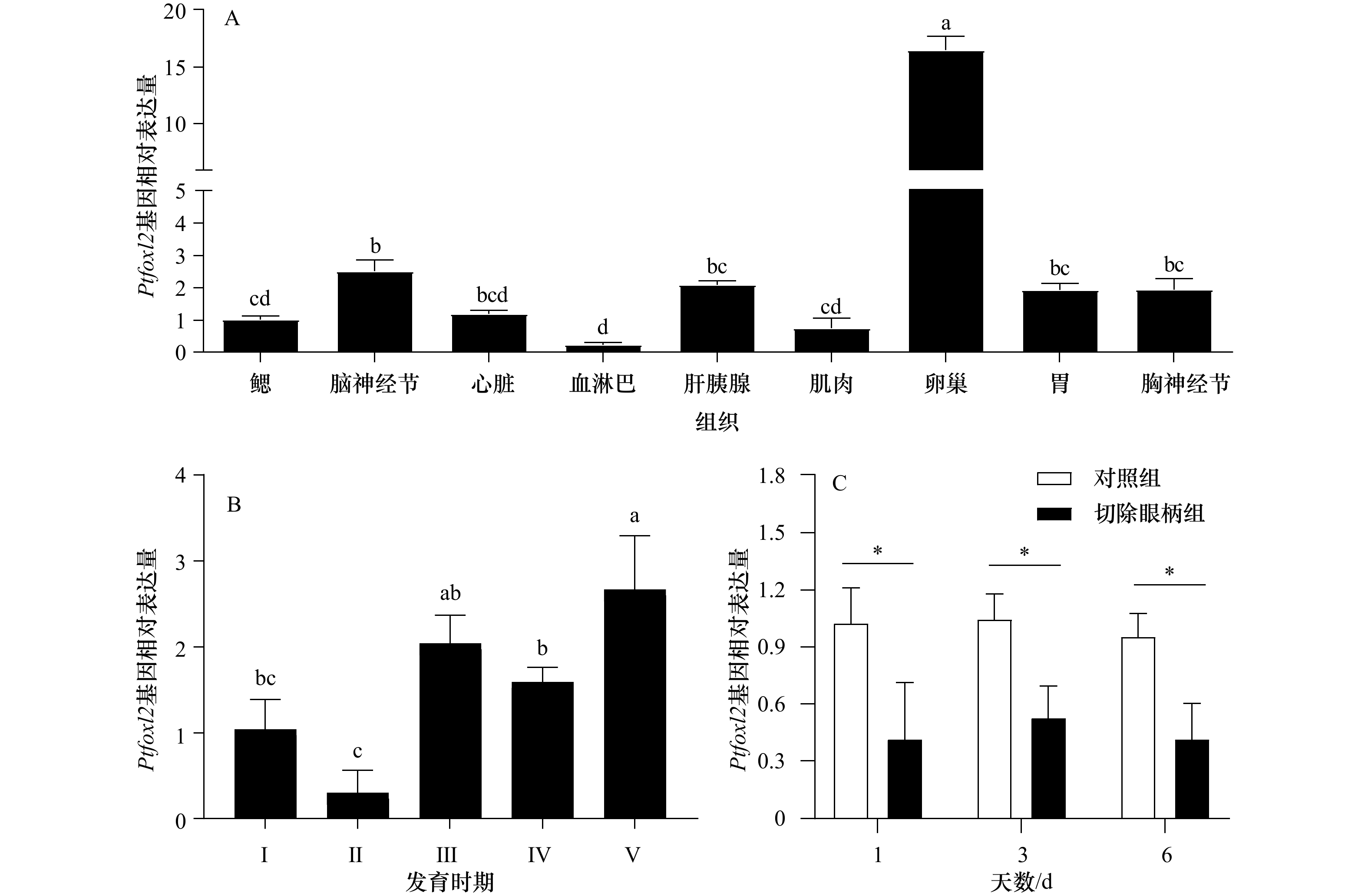
图 3 Ptfoxl2基因在三疣梭子蟹不同组织(A)、不同卵巢发育时期(B)以及切除眼柄后(C)的表达
不同字母代表数据差异显著(p<0.05);*表示对照组和切除眼柄组表达存在显著差异(p<0.05)
Fig. 3 The expression of Ptfoxl2 in different tissues (A), in different ovarian developmental stages (B) and after eyestalk ablation (C)
Different letters indicate significant difference (p<0.05); * indicates a significant difference between the control group and eyestalk ablation group (p<0.05)
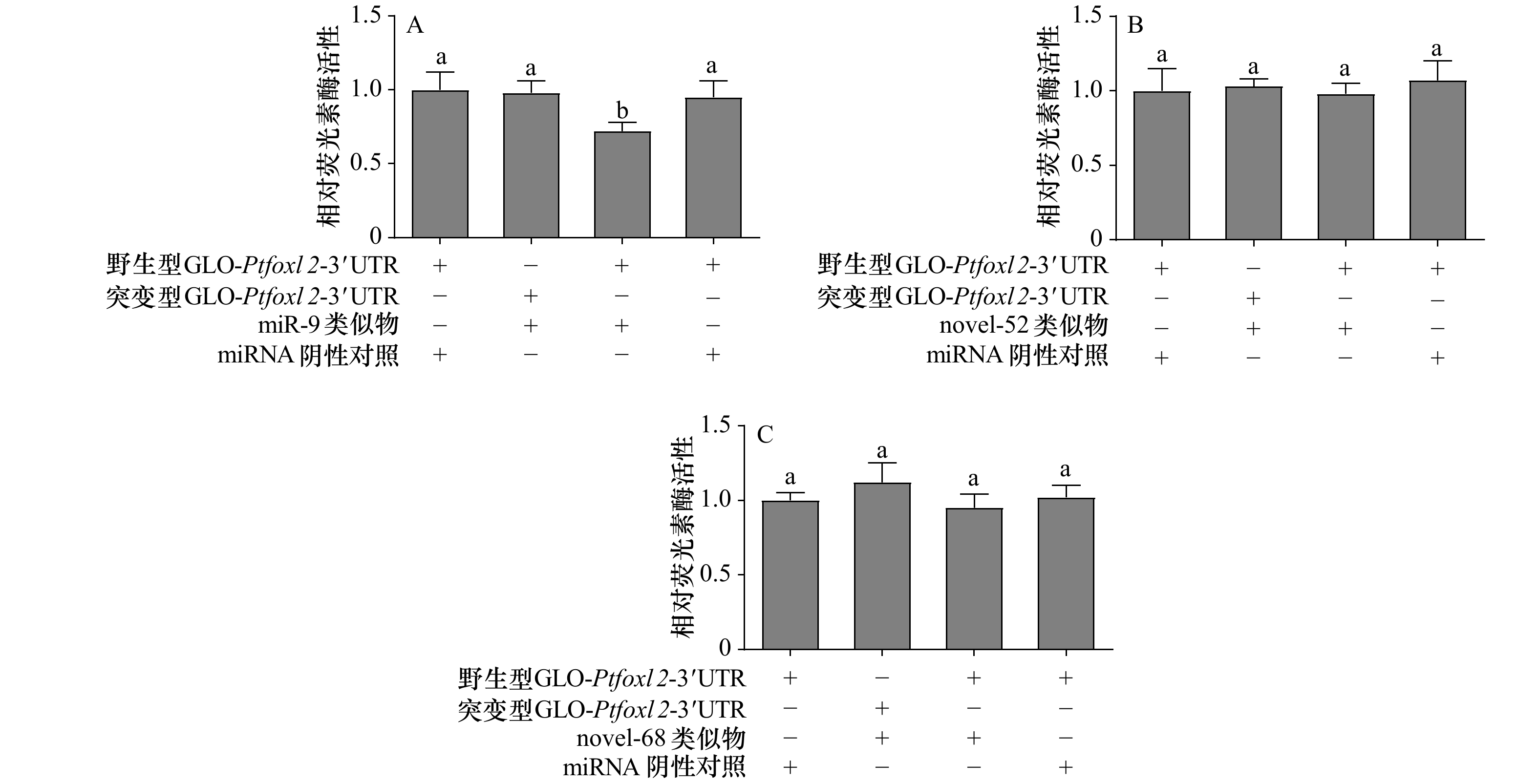
图 5 共转染野生型和突变型质粒和miRNA类似物后荧光素酶的相对活性
+表示实验体系中存在该miRNA类似物或质粒;−表示实验体系中不存在该miRNA类似物或质粒;不同字母代表数据差异显著(p<0.05)
Fig. 5 Luciferase activity of the reporter plasmid containing wild-type or mutant target site after co-transfection of plasmid and miRNA mimics
+ indicates that the miRNA mimics or plasmid exists in the reaction system; − indicates that the miRNA mimics or plasmid doesn’t exist in the reaction system; different letters indicate significant difference (p<0.05)
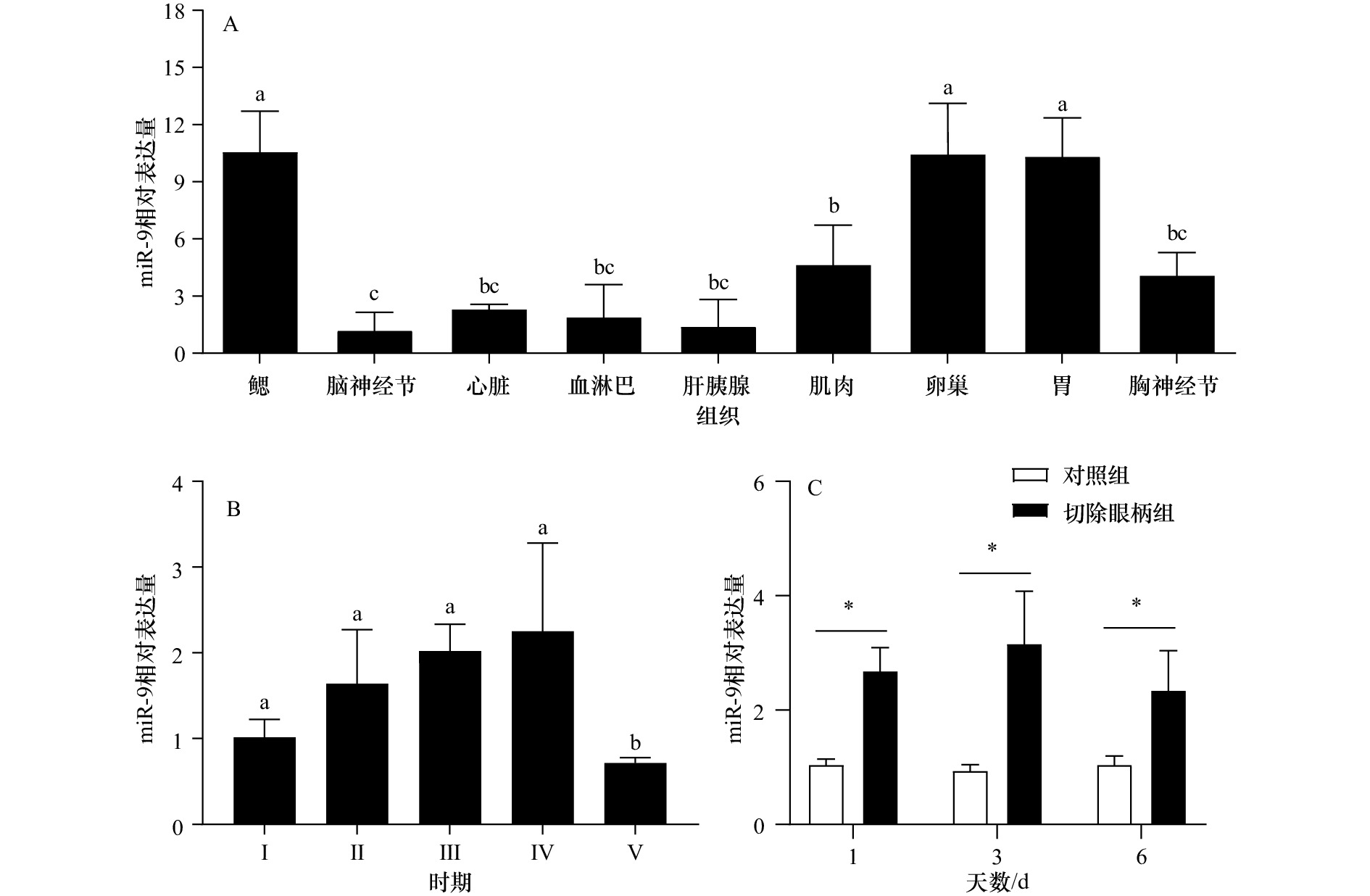
图 6 miR-9在三疣梭子蟹不同组织(A)、不同卵巢发育时期(B)以及切除眼柄后(C)的表达
不同字母代表数据差异显著(p<0.05);*表示对照组和切除眼柄组表达存在显著差异(p<0.05)
Fig. 6 The expression of miR-9 in different tissues (A), in different ovarian developmental stages (B) and after eyestalk ablation (C)
Different letters indicate significant difference (p<0.05) ; * indicates a significant difference between the control group and eyestalk ablation group (p<0.05)
表 1 实验所用引物的序列
Tab. 1 The sequences of the primers used in this study
引物名称 序列(5′-3′) 用途 foxl2-3′ CGGGAACTTCGCAAGCTACACACAG RACE foxl2-5′ GGTAGTCGTCTTTCATCGTGCCGTAGG RACE UPM CTAATACGACTCACTATAGGGC RACE foxl2-F CGTTGTCCTGATCTCACTGC qRT-PCR foxl2-R CGTCTTTCATCGTGCCGTAG qRT-PCR β-actin-F CGAAACCTTCAACACTCCCG qRT-PCR β-actin-R GGGACAGTGTGTGAAACGCC qRT-PCR dsRNA-foxl2-T7-F GGATCCTAATACGACTCACTATAGGGCAGATGCAAAGCGGGAACTT RNAi dsRNA-foxl2-T7-R GGATCCTAATACGACTCACTATAGGGGGAAAGCGTCTCCAGTCATC RNAi dsRNA-GFP-T7-F GGATCCTAATACGACTCACTATAGGCGACGTAAACGGCCACAAGTT RNAi dsRNA-GFP-T7-R GGATCCTAATACGACTCACTATAGGATGGGGGTGTTCTGCTGGTAG RNAi miR-9 GCCTCTTTGGTTATCTAGCTGTAT qRT-PCR -
[1] Cocquet J, Pailhoux E, Jaubert F, et al. Evolution and expression of FOXL2[J]. Journal of Medical Genetics, 2002, 39(12): 916−921. doi: 10.1136/jmg.39.12.916 [2] De Baere E, Lemercier B, Christin-Maitre S, et al. FOXL2 mutation screening in a large panel of POF patients and XX males[J]. Journal of Medical Genetics, 2002, 39(8): e43−e43. doi: 10.1136/jmg.39.8.e43 [3] Crisponi L, Deiana M, Loi A, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome[J]. Nature Genetics, 2001, 27(2): 159−166. doi: 10.1038/84781 [4] Schmidt D, Ovitt C E, Anlag K, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance[J]. Development, 2004, 131(4): 933−942. doi: 10.1242/dev.00969 [5] Uda M, Ottolenghi C, Crisponi L, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development[J]. Human Molecular Genetics, 2004, 13(11): 1171−1181. doi: 10.1093/hmg/ddh124 [6] Uhlenhaut N H, Jakob S, Anlag K, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation[J]. Cell, 2009, 139(6): 1130−1142. doi: 10.1016/j.cell.2009.11.021 [7] Moumné L, Batista F, Benayoun B A, et al. The mutations and potential targets of the forkhead transcription factor FOXL2[J]. Molecular and Cellular Endocrinology, 2008, 282(1/2): 2−11. [8] Baron D, Cocquet J, Xia Xuhua, et al. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation[J]. Journal of Molecular Endocrinology, 2004, 33(3): 705−715. doi: 10.1677/jme.1.01566 [9] Wang W C, Lai Y C. Molecular pathogenesis in granulosa cell tumor is not only due to somatic FOXL2 mutation[J]. Journal of Ovarian Research, 2014, 7(1): 88. [10] Nakamoto M, Matsuda M, Wang Deshou, et al. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes[J]. Biochemical and Biophysical Research Communications, 2006, 344(1): 353−361. doi: 10.1016/j.bbrc.2006.03.137 [11] Oshima Y, Uno Y, Matsuda Y, et al. Molecular cloning and gene expression of Foxl2 in the frog Rana rugosa[J]. General and Comparative Endocrinology, 2008, 159(2/3): 170−177. [12] Shi Bao, Wen H S, He Feng, et al. Association of reproductive performance with SNPs of FOXL2 gene by SSCP in Japanese flounder (Paralichthys olivaceus)[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2009, 153(1): 1−7. doi: 10.1016/j.cbpb.2008.10.007 [13] Uhlenhaut N H, Treier M. Foxl2 function in ovarian development[J]. Molecular Genetics and Metabolism, 2006, 88(3): 225−234. doi: 10.1016/j.ymgme.2006.03.005 [14] 王雪芹. 稀有鮈鲫Foxl2基因的克隆及内分泌干扰物对其表达的影响[D]. 杨凌: 西北农林科技大学, 2013.Wang Xueqin. Molecular cloning and characterrization of foxl2 gene and its response to endocrine disrupting chemicals in rare minnow[D]. Yangling: Northwest A&F University, 2013. [15] 陈玲玲, 冯珊珊, 范祖森, 等. 非编码RNA研究进展[J]. 中国科学: 生命科学, 2019, 49(12): 1573−1605.Chen Lingling, Feng Shanshan, Fan Zusen, et al. Progress in non-coding RNA research[J]. Science China Life Sciences, 2019, 49(12): 1573−1605. [16] 贺小云, 刘秋月, 储明星. miRNA调控哺乳动物卵泡发育和卵母细胞成熟的研究进展[J]. 畜牧兽医学报, 2019, 50(11): 2175−2185. doi: 10.11843/j.issn.0366-6964.2019.11.001He Xiaoyun, Liu Qiuyue, Chu Mingxing. Advances in miRNA regulating mammalian follicular development and oocyte maturation[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(11): 2175−2185. doi: 10.11843/j.issn.0366-6964.2019.11.001 [17] Zhou Mingcan, Jia Xiwei, Wan Haifu, et al. miR-9 and miR-263 regulate the key genes of the ERK pathway in the ovary of mud crab scylla paramamosain[J]. Marine Biotechnology, 2020, 22(4): 594−606. doi: 10.1007/s10126-020-09981-4 [18] Wan Haifu, Zhong Jinying, Zhang Ziping, et al. Characterization of the foxl2 gene involved in the vtg expression in mud crab (Scylla paramamosain)[J]. Gene, 2021, 798: 145807. doi: 10.1016/j.gene.2021.145807 [19] 农业农村部渔业渔政管理局. 中国渔业统计年鉴[M]. 北京: 中国农业出版, 2019.Fisheries Administration of Ministry of Agriculture and Rural Affairs. China Fishery Statistical Yearbook 2019[M]. Beijing: China Agriculture Press, 2019. [20] Meng Xianliang, Liu Ping, Jia Fulong, et al. De novo transcriptome analysis of Portunus trituberculatus ovary and testis by RNA-Seq: identification of genes involved in gonadal development[J]. PLoS One, 2015, 10(6): e0128659. doi: 10.1371/journal.pone.0128659 [21] 吴旭干, 姚桂桂, 杨筱珍, 等. 东海三疣梭子蟹第一次卵巢发育规律的研究[J]. 海洋学报, 2007, 29(4): 120−127.Wu Xugan, Yao Guigui, Yang Xiaozhen, et al. A study on the ovarian development of Portunus trituberculatus in East China Sea during the first reproductive cycle[J]. Acta Oceanologica Sinica, 2007, 29(4): 120−127. [22] Liu Xiaoling, Zhang Zhifeng, Shao Mingyu, et al. Sexually dimorphic expression of foxl2 during gametogenesis in scallop Chlamys farreri, conserved with vertebrates[J]. Development Genes and Evolution, 2012, 222(5): 279−286. doi: 10.1007/s00427-012-0410-z [23] Wei Huilan, Li Wanru, Liu Tian, et al. Sexual development of the hermaphroditic scallop Argopecten irradians revealed by morphological, endocrine and molecular analysis[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 646754. doi: 10.3389/fcell.2021.646754 [24] Browdy C L, Samocha T M. The effect of eyestalk ablation on spawning, molting and mating of Penaeus semisulcatus de Haan[J]. Aquaculture, 1985, 49(1): 19−29. doi: 10.1016/0044-8486(85)90187-5 [25] Uawisetwathana U, Leelatanawit R, Klanchui A, et al. Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp[J]. PloS One, 2011, 6(9): e24427. doi: 10.1371/journal.pone.0024427 [26] 贾复龙, 孟宪亮, 刘萍, 等. 三疣梭子蟹细胞Cdk7基因克隆及其在卵巢发育中的表达[J]. 中国水产科学, 2016, 23(5): 1032−1040.Jia Fulong, Meng Xianliang, Liu Ping, et al. Cloning and expression analysis of Cdk7, a gene involved in ovarian development, from swimming crab (Portunus trituberculatus)[J]. Journal of Fishery Sciences of China, 2016, 23(5): 1032−1040. [27] 贾复龙, 孟宪亮, 刘萍, 等. 三疣梭子蟹细胞周期蛋白H基因克隆及其在卵巢发育中的表达分析[J]. 中国海洋大学学报, 2016, 46(7): 62−69.Jia Fulong, Meng Xianliang, Liu Ping, et al. Clong and expression in the ovarian development of Cyclin H gene of Portunus trituberculatus[J]. Periodical of Ocean University of China, 2016, 46(7): 62−69. [28] Meng Xianliang, Zhang Mengqian, Gao Baoquan, et al. Integrative proteomic and microrna analysis: insights into mechanisms of eyestalk ablation-induced ovarian maturation in the swimming crab Portunus trituberculatus[J]. Frontiers in Endocrinology, 2020, 11: 533. doi: 10.3389/fendo.2020.00533 [29] Song Ya’nan, Shi Lili, Liu Zhiqiang, et al. Global analysis of the ovarian microRNA transcriptome: implication for miR-2 and miR-133 regulation of oocyte meiosis in the Chinese mitten crab, Eriocheir sinensis (Crustacea: Decapoda)[J]. BMC Genomics, 2014, 15(1): 547. doi: 10.1186/1471-2164-15-547 [30] Jia Xiwei, Fang Zhiqiang, Zeng Xianyuan, et al. Regulation of VIH by miR-277 in the eyestalk of mud crab Scylla paramamosain[J]. Aquaculture, 2021, 534: 736254. doi: 10.1016/j.aquaculture.2020.736254 [31] 张小辉. MiRNA及其合成通路相关基因在三疣梭子蟹性腺发育过程中的功能分析[D]. 上海: 上海海洋大学, 2017.Zhang Xiaohui. Functional analysis of miRNA and the genes in the miRNA biogenesis pathway in gonadal development of Portunus trituberculatus[D]. Shanghai: Shanghai Ocean University, 2017. -





 下载:
下载: