Physiological responses of juvenile Apostichopus japonicus (Selenka) to collaborative stress of high temperature and low salinity: Growth and induced heat shock protein gene expression
-
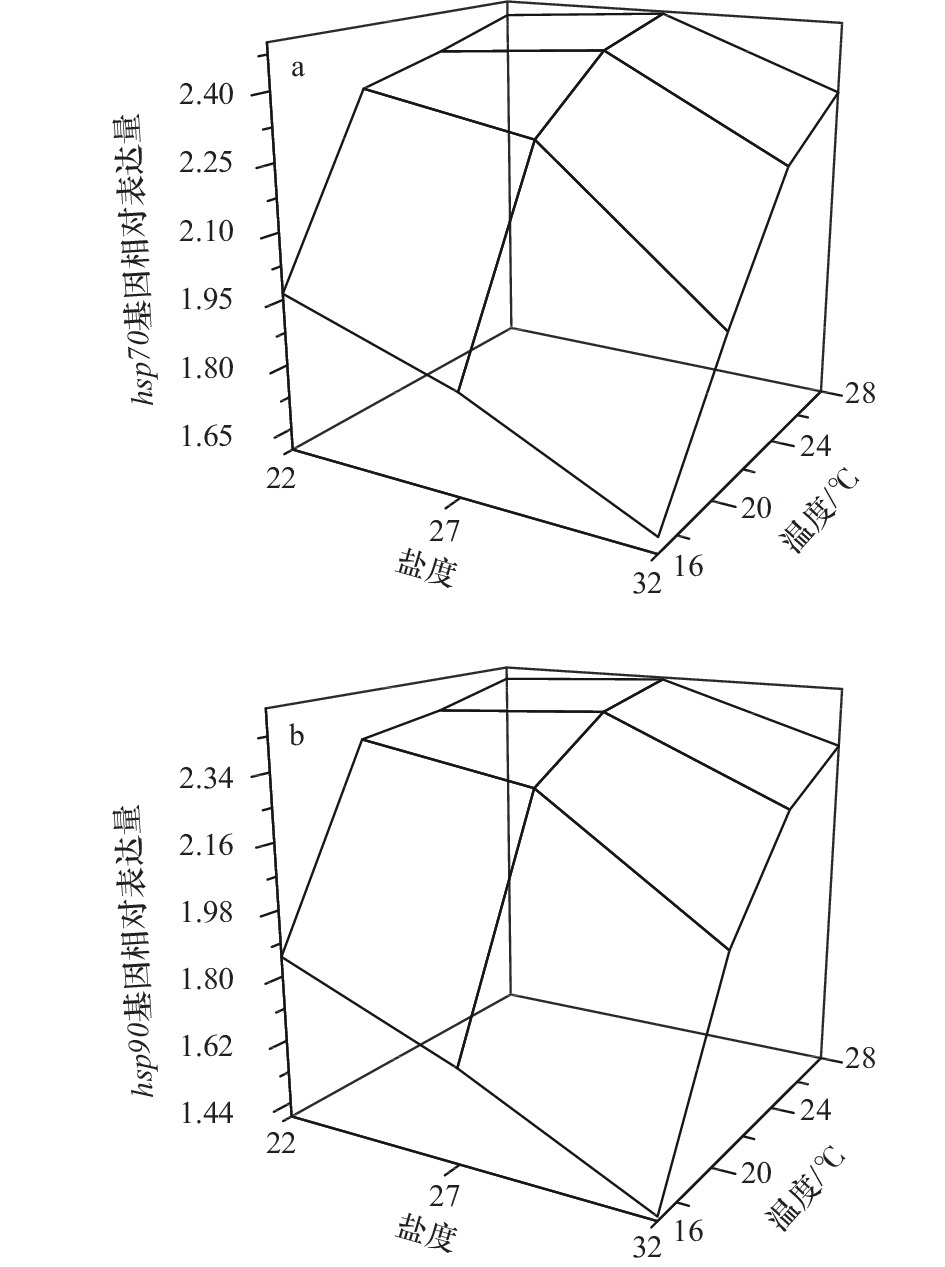
摘要: 全球气候变化背景下的极端高温和强降雨频发天气导致的养殖池塘持续性的高温和低盐环境给刺参池塘养殖带来了严峻的挑战。为了研究刺参对高温和低盐环境的生理响应,本实验分析了高温和低盐协同胁迫对刺参幼参生长及诱导型hsp70和hsp90基因表达的影响。实验设置4个温度梯度(16℃、20℃、24℃和28℃)和3个盐度梯度(22、27和32),共12个不同的胁迫组。经过40 d的长期胁迫,研究发现,随着温度的升高和盐度的降低,幼参的体重增加量减少,并在高温、低盐组出现体重负增长。长期胁迫提高了刺参幼参的诱导型hsp70和hsp90基因表达量,从而在一定程度上增强了对极端天气的抵抗能力。同时,高温下盐度22处理组刺参幼参诱导型hsp70和hsp90基因的表达量较盐度27条件下低。双因子方差分析结果显示,高温和低盐对幼参特定生长率和诱导型hsp70和hsp90基因表达量的交互作用均不显著,并且盐度对诱导型hsp70和hsp90基因表达量没有显著的影响。因此,相较低盐,高温对刺参幼参的影响更大,可作为刺参育种的选择压力。在高温期应该采取有效措施降低海水温度,并防止极端天气造成的养殖水体温度分层。该研究丰富了刺参生理生态学理论,可为刺参良种选育工作提供思路,并为指导极端天气下刺参生产实践活动提供一定的理论依据。Abstract: Under the background of global climate change, the continuous high temperature and low salinity caused by extreme short-time heavy rainfall in summer brings severe challenges to pond culture of Apostichopus japonicus. In order to study the physiological responses of A. japonicus to high temperature and low salinity, the effects of collaborative stress of high temperature and low salt on growth and induced hsp70 and hsp90 expression of juvenile A. japonicus were analyzed in this paper. Four temperature gradients (16℃, 20℃, 24℃, and 28℃) and three salinity gradients (22, 27, and 32) were set, and there were a total of 12 different stress groups. After a period of 40 days stress, the weight gain of juvenile A. japonicus decreased with the increase of temperature and the decrease of salinity, there was negative growth in the high temperature and low salinity group. The expression of induced hsp70 and hsp90 was increased after a long period of stress, thus enhancing the resistance of juveniles to extreme weather. Meanwhile, the expression of induced hsps of juveniles treated with salinity 22 was lower than that in the condition of salinity 27. The results of two-factor ANOVA showed that there was no significant interaction between high temperature and low salinity on specific growth rate and induced hsp70 and hsp90 expression, and salinity had no significant effect on induced hsp70 and hsp90 expression. Therefore, compared with low salinity, high temperature had a greater impact on juvenile A. japonicus, which could be used as the selection pressure in A. japonicus breeding. During the high temperature period, effective measures should be taken to reduce the sea temperature and prevent the stratification of aquaculture water caused by extreme weather. This study enriched the physiological and ecological theories of A. japonicus, provided ideas for the breeding of A. japonicus, and provided certain theoretical basis for guiding the production and practice of A. japonicus culture under extreme weather.
-
Key words:
- high temperature /
- low salinity /
- collaborative stress /
- Apostichopus japonicus /
- growth /
- heat shock protein
-
图 1 高温和低盐协同胁迫对刺参幼参特定生长率的影响
小写字母不同表示同一盐度下不同温度处理组差异显著(p<0.05);大写字母表示同一温度下不同盐度处理组差异显著(p<0.05)
Fig. 1 Effects of high temperature and low salinity collaborative stress on specific growth rate of juvenile Apostichopus japonicus
Data in the same row having different lower case letters are significantly different (p<0.05) among different temperature levels; data in the same column having different capital letters are significantly different (p<0.05) among different salinity levels
表 1 用于本实验的引物序列
Tab. 1 Primers used in this study
引物名称 引物序列 Hsp70-F 5’-ATGCCTAGAACCAGTAGAGAAAG-3’ Hsp70-R 5’-TGTCGTTCGTGATGGTGATT-3’ Hsp90-F 5’-TTGTTGAAAGGGAGGAGG-3’ Hsp90-R 5’-GGCATCAGAGGCGTTAGA-3’ β-actin-F 5’-ACACGGTATCGTCACAAACTGG-3’ β-actin-R 5’-AGGATAGCGTGAGGAAGAGCAT-3’ 表 2 高温和低盐协同胁迫对刺参幼参生长的影响
Tab. 2 Effects of high temperature and low salinity collaborative stress on growth of juvenile Apostichopus japonicus
温度/℃ 盐度 初体重/g 末体重/g 个体数 16 22 3.574±0.024a 5.264±0.263c 18 16 27 3.543±0.029a 7.717±0.413b 18 16 32 3.544±0.033a 10.618±0.782a 18 20 22 3.549±0.025a 4.278±0.274c 18 20 27 3.530±0.015a 5.239±0.261c 18 20 32 3.558±0.029a 7.290±0.582b 18 24 22 3.507±0.012a 2.765±0.149de 18 24 27 3.534±0.034a 3.963±0.147c 18 24 32 3.555±0.049a 5.077±0.166c 18 28 22 3.525±0.036a 2.089±0.047e 18 28 27 3.553±0.007a 2.429±0.075e 18 28 32 3.520±0.032a 3.898±0.094cd 18 注:同一列上标字母不同表示差异显著(p<0.05)。 表 3 温度和盐度对刺参幼参生长的双因子方差分析
Tab. 3 Two-way ANOVA of growth for the juvenile Apostichopus japonicus maintained in different temperature and salinity
影响因子 自由度 均方 F值 p 末体重/g 温度/℃ 3 2.094 172.761 < 0.001 盐度 2 1.389 114.546 < 0.001 交互作用 6 0.035 2.917 < 0.05 特定生长率 温度/℃ 3 10.894 220.109 < 0.001 盐度 2 7.100 143.439 < 0.001 交互作用 6 0.093 1.874 > 0.05 表 4 温度和盐度对刺参幼参诱导型hsp基因表达量的双因子方差分析
Tab. 4 Two-way ANOVA of hsp gene expression for the juvenile Apostichopus japonicus maintained in different temperature and salinity levels
影响因子 自由度 均方 F值 p hsp70 温度/℃ 3 0.694 7.207 < 0.05 盐度 2 0.241 2.502 > 0.05 交互作用 6 0.015 0.159 > 0.05 hsp90 温度/℃ 3 1.472 8.541 < 0.001 盐度 2 0.321 1.861 > 0.05 交互作用 6 0.027 0.157 > 0.05 -
[1] Shao Yina, Li Chenghua, Chen Xiaocong, et al. Metabolomic responses of sea cucumber Apostichopus japonicus to thermal stresses[J]. Aquaculture, 2015, 435: 390−397. doi: 10.1016/j.aquaculture.2014.10.023 [2] Purcell S W, Conand C, Uthicke S, et al. Ecological roles of exploited sea cucumbers[J]. Oceanography and Marine Biology: An Annual Review, 2016, 54: 367−386. [3] Ding Kui, Zhang Libin, Sun Lina, et al. Transcriptome analysis provides insights into the molecular mechanisms responsible for evisceration behavior in the sea cucumber Apostichopus japonicus[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomic, 2019, 30: 143−157. [4] Huo Da, Sun Lina, Zhang Libin, et al. Global-warming-caused changes of temperature and oxygen alter the proteomic profile of sea cucumber Apostichopus japonicus[J]. Journal of Proteomics, 2019, 193: 27−43. doi: 10.1016/j.jprot.2018.12.020 [5] 杨红生, 孙景春, 茹小尚, 等. 我国刺参种业态势分析与技术创新展望[J]. 海洋科学, 2020, 44(7): 2−9.Yang Hongsheng, Sun Jingchun, Ru Xiaoshang, et al. Current advances and technological prospects of the sea cucumber seed industry in China[J]. Marine Sciences, 2020, 44(7): 2−9. [6] 农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2021中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2021: 15-84.Fishery Administration of the Ministry of Agriculture and Rural Areas, National Aquatic Technology Promotion Station, China Fisheries Society. China Fishery Statistical Yearbook[M]. Beijing: China Agriculture Press, 2021: 15−84. [7] Huo Da, Sun Lina, Zhang Libin, et al. Time course analysis of immunity-related gene expression in the sea cucumber Apostichopus japonicus during exposure to thermal and hypoxic stress[J]. Fish & Shellfish Immunology, 2019, 95: 383−390. [8] Tian Yi, Shang Yanpeng, Guo Ran, et al. Salinity stress-induced differentially expressed miRNAs and target genes in sea cucumbers Apostichopus japonicus[J]. Cell Stress and Chaperones, 2019, 24(4): 719−733. [9] Yang Hongsheng, Yuan Xiutang, Zhou Yi, et al. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation[J]. Aquaculture Research, 2005, 36(11): 1085−1092. doi: 10.1111/j.1365-2109.2005.01325.x [10] Wang Qinglin, Yu Shanshan, Dong Yunwei. Parental effect of long acclimatization on thermal tolerance of juvenile sea cucumber Apostichopus japonicus[J]. PLoS One, 2015, 10(11): e0143372. doi: 10.1371/journal.pone.0143372 [11] Liu Shilin, Zhang Sicong, Ru Xiaoshang, et al. Effect of high temperature stress on the fertility of male and female gametes of the sea cucumber Apostichopus japonicus[J]. Aquaculture Research, 2016, 47(10): 3127−3135. doi: 10.1111/are.12763 [12] Yu Haibo, Zhang Cheng, Gao Qinfeng, et al. Impact of water temperature on the growth and fatty acid profiles of juvenile sea cucumber Apostichopus japonicus (Selenka)[J]. Journal of Thermal Biology, 2016, 60: 155−161. doi: 10.1016/j.jtherbio.2016.07.011 [13] Bai Yucen, Chen Yan, Pan Yang, et al. Effect of temperature on growth, energy budget, and physiological performance of green, white, and purple color morphs of sea cucumber, Apostichopus japonicus[J]. Journal of the World Aquaculture Society, 2018, 49(3): 625−637. doi: 10.1111/jwas.12505 [14] Geng Chenfan, Tian Yi, Shang Yanpeng, et al. Effect of acute salinity stress on ion homeostasis, Na+/K+-ATPase and histological structure in sea cucumber Apostichopus japonicus[J]. SpringerPlus, 2016, 5(1): 1977. doi: 10.1186/s40064-016-3620-4 [15] 袁秀堂, 杨红生, 周毅, 等. 盐度对刺参(Apostichopus japonicus)呼吸和排泄的影响[J]. 海洋与湖沼, 2006, 37(4): 348−354. doi: 10.3321/j.issn:0029-814X.2006.04.010Yuan Xiutang, Yang Hongsheng, Zhou Yi, et al. Salinity effect on respiration and excretion of sea cucumber Apostichopus japonicus (Selenka)[J]. Oceanologia et Limnologia Sinica, 2006, 37(4): 348−354. doi: 10.3321/j.issn:0029-814X.2006.04.010 [16] 胡炜, 李成林, 赵斌, 等. 低盐胁迫对刺参存活、摄食和生长的影响[J]. 渔业科学进展, 2012, 33(2): 92−96. doi: 10.3969/j.issn.1000-7075.2012.02.014Hu Wei, Li Chenglin, Zhao Bin, et al. Effects of low salinity stress on survival, growth and feeding rate of sea cucumber Apostichopus japonicus[J]. Progress in Fishery Sciences, 2012, 33(2): 92−96. doi: 10.3969/j.issn.1000-7075.2012.02.014 [17] Wang Qinglin, Yu Shanshan, Qin Chuanxin, et al. Combined effects of acute thermal and hypo-osmotic stresses on osmolality and hsp70, hsp90 and sod expression in the sea cucumber Apostichopus japonicus Selenka[J]. Aquaculture International, 2014, 22(3): 1149−1161. doi: 10.1007/s10499-013-9734-6 [18] Song Lin, Li Chao, Xie Yangjie, et al. Genome-wide identification of Hsp70 genes in channel catfish and their regulated expression after bacterial infection[J]. Fish & Shellfish Immunology, 2016, 49: 154−162. [19] Morimoto R I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators[J]. Genes & Development, 1998, 12(24): 3788−3796. [20] Li Jiuxuan, Zhang Haibin, Zhang Xiuyue, et al. Molecular cloning and expression of two heat-shock protein genes (HSC70/HSP70) from Prenant's schizothoracin (Schizothorax prenanti)[J]. Fish Physiology and Biochemistry, 2015, 41(2): 573−585. doi: 10.1007/s10695-015-0030-4 [21] Xu Dongxue, Sun Lina, Liu Shilin, et al. Histological, ultrastructural and heat shock protein 70 (HSP70) responses to heat stress in the sea cucumber Apostichopus japonicus[J]. Fish & Shellfish Immunology, 2015, 45(2): 321−326. [22] Wang Qinglin, Dong Yunwei, Qin Chuanxin, et al. Effects of rearing temperature on growth, metabolism and thermal tolerance of juvenile sea cucumber, Apostichopus japonicus Selenka: critical thermal maximum (CTmax) and hsps gene expression[J]. Aquaculture Research, 2013, 44(10): 1550−1559. doi: 10.1111/j.1365-2109.2012.03162.x [23] Han Guodong, Cartwright S R, Ganmanee M, et al. High thermal stress responses of Echinolittorina snails at their range edge predict population vulnerability to future warming[J]. Science of the Total Environment, 2019, 647: 763−771. doi: 10.1016/j.scitotenv.2018.08.005 [24] Palumbi S R, Barshis D J, Traylor-Knowles N, et al. Mechanisms of reef coral resistance to future climate change[J]. Science, 2014, 344(6186): 895−898. doi: 10.1126/science.1251336 [25] O’Leary B C, Houghton D C, Van Zant J. Differences in thermal tolerance between two thermally isolated and genetically indistinct populations of Paragnetina Media (Walker) (Plecoptera: Perlodidae)[J]. The Great Lakes Entomologist, 2018, 47(3/4): 101−113. [26] Hu Meiyan, Li Qi, Li Li. Effect of salinity and temperature on salinity tolerance of the sea cucumber Apostichopus japonicus[J]. Fishery Science, 2010, 76(2): 267−273. doi: 10.1007/s12562-010-0214-x [27] Eissa N, Wang Hanping, Yao Hong, et al. Expression of hsp70, igf1, and three oxidative stress biomarkers in response to handling and salt treatment at different water temperatures in Yellow Perch, Perca flavescens[J]. Frontiers in Physiology, 2017, 8: 683. doi: 10.3389/fphys.2017.00683 [28] Fang Zhiqiang, Sun Yulong, Zhang Xin, et al. Responses of HSP70 gene to Vibrio parahaemolyticus infection and thermal stress and its transcriptional regulation analysis in Haliotis diversicolor[J]. Molecules, 2019, 24(1): 162. doi: 10.3390/molecules24010162 [29] 包鹏云, 周德刚, 蒲红宇. 我国海参池塘养殖存在的问题及应对措施[J]. 科学养鱼, 2011(3): 3−5.Bao Pengyun, Zhou Degang, Pu Hongyu. Existing problems and solutions of pond-cultured sea cucumber in China[J]. Scientific Fish Farming, 2011(3): 3−5. [30] Li Xishan, Liao Guoxiang, Ju Zhonglei, et al. Antioxidant response and oxidative stress in the respiratory tree of sea cucumber (Apostichopus japonicus) following exposure to crude oil and chemical dispersant[J]. Journal of Marine Science and Engineering, 2020, 8(8): 547. doi: 10.3390/jmse8080547 [31] Zhang Libin, Feng Qiming, Sun Lina, et al. Differential gene expression in the intestine of sea cucumber (Apostichopus japonicus) under low and high salinity conditions[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2018, 25: 34−41. doi: 10.1016/j.cbd.2017.11.001 [32] 赵斌, 胡炜, 李成林, 等. 温度和盐度对紫刺参(Apostichopus japonicus Selenta)稚参存活、生长和着色的影响[J]. 海洋与湖沼, 2018, 49(3): 700−706.Zhao Bin, Hu Wei, Li Chenglin, et al. Effects of temperature and salinity on survival, growth, and coloration of juvenile Apostichopus japonicus Selenta[J]. Oceanologia et Limnologia Sinica, 2018, 49(3): 700−706. [33] 王欠欠, 杨建敏, 王腾腾, 等. 不同温度下低盐度对仿刺参生长的影响[J]. 海洋湖沼通报, 2018(3): 157−161.Wang Qianqian, Yang Jianmin, Wang Tengteng, et al. Effect of low salinity on the growth of sea cucumber Apostichopus japonicus at different temperatures[J]. Transactions of Oceanology and Limnology, 2018(3): 157−161. [34] Cahan S H, Nguyen A D, Stanton-Geddes J, et al. Modulation of the heat shock response is associated with acclimation to novel temperatures but not adaptation to climatic variation in the ants Aphaenogaster picea and A. rudis[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2017, 204: 113−120. [35] Hupało K, Riss H W, Grabowski M, et al. Climate change as a possible driver of invasion and differential in HSP70 expression in two genetically distinct populations of the invasive killer shrimp, Dikerogammarus villosus[J]. Biological Invasions, 2018, 20(8): 2047−2059. doi: 10.1007/s10530-018-1679-2 [36] Mascaró M, Amaral-Ruiz M, Huipe-Zamora I, et al. Thermal tolerance and phenotypic plasticity in juvenile Hippocampus erectus Perry, 1810: effect of acute and chronic exposure to contrasting temperatures[J]. Journal of Experimental Marine Biology and Ecology, 2016, 483: 112−119. doi: 10.1016/j.jembe.2016.07.005 [37] Machado A, Cardoso C M, Sartorio P V, et al. Lethal thermal maximum temperature induces behavioral responses and protein expressions (Hsp70 and p53) in juvenile common carp (Cyprinus carpio Linnaeus)[J]. Pan-American Journal of Aquatic Sciences, 2017, 12(4): 295−309. [38] 俞丹, 沈中源, 张智, 等. 温度驯化对尖头鳉热耐受特征的影响[J]. 水生生物学报, 2017, 41(3): 538−542. doi: 10.7541/2017.69Yu Dan, Shen Zhongyuan, Zhang Zhi, et al. Effect of temperature acclimation on the thermal tolerance of Rhynchocypris oxycephalus[J]. Acta Hydrobiologica Sinica, 2017, 41(3): 538−542. doi: 10.7541/2017.69 [39] Lai J J, Hou P C L, Steinberger Y. Thermal plasticity in the burst swimming of Bufo bankorensis larvae[J]. Ecology and Sustainable Development, 2018, 1(2): 57−68. [40] Dong Yunwei, Williams G A. Variations in cardiac performance and heat shock protein expression to thermal stress in two differently zoned limpets on a tropical rocky shore[J]. Marine Biology, 2011, 158(6): 1223−1231. doi: 10.1007/s00227-011-1642-6 [41] Wang Jie, Russell B D, Ding M W, et al. Ocean acidification increases the sensitivity of and variability in physiological responses of an intertidal limpet to thermal stress[J]. Biogeosciences, 2018, 15(9): 2803−2817. doi: 10.5194/bg-15-2803-2018 [42] Sung Y Y, Liew H J, Bolong A M A, et al. The induction of Hsp70 synthesis by non-lethal heat shock confers thermotolerance and resistance to lethal ammonia stress in the common carp, Cyprinus carpio (Linn)[J]. Aquaculture Research, 2013, 45(10): 1706−1712. [43] Wali A, Balkhia M H. Heat shock proteins, importance and expression in fishes[J]. European Journal of Biotechnology and Bioscience, 2016, 4(4): 29−35. [44] Guerriero G, Bassem S M, Khalil W K B, et al. Temperature changes and marine fish species (Epinephelus coioides and Sparus aurata): role of oxidative stress biomarkers in toxicological food studies[J]. Emirates Journal of Food and Agriculture, 2018, 30(3): 205−211. [45] Finka A, Sood V, Quadroni M, et al. Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs[J]. Cell Stress and Chaperones, 2015, 20(4): 605−620. doi: 10.1007/s12192-015-0583-2 [46] 董云伟, 董双林. 刺参对温度适应的生理生态学研究进展[J]. 中国海洋大学学报(自然科学版), 2009, 39(5): 908−912.Dong Yunwei, Dong Shuanglin. Advances of ecological physiology in sea cucumber, Apostichopus japonicus Selenka[J]. Periodical of Ocean University of China, 2009, 39(5): 908−912. -





 下载:
下载: