Identification of genes related to blind side hypermelanosis of Cynoglossus semilaevis based on skin transcriptome sequencing
-
摘要: 半滑舌鳎(Cynoglossus semilaevis)作为比目鱼(Pleuronectiformes)中的代表物种,是我国沿海地区重要的海水经济鱼类。半滑舌鳎体色异常的问题长期以来一直困扰着从业者。导致体色变化的原因多种多样,遗传因素是最根本原因。针对半滑舌鳎无眼侧黑化的研究多在环境、营养、生理、常见色素基因克隆等方面展开,新的功能基因挖掘工作欠缺。本研究选取半滑舌鳎不同体色皮肤进行转录组测序,通过差异基因GO和KEGG富集比对分析,在可能的6条黑色素相关的KEGG通路中筛查差异基因并对差异表达最为显著的10个基因进行了实时荧光定量聚合酶链式反应的验证。本研究找出了半滑舌鳎无眼侧黑化表达显著变化的5个功能基因,分别为txndc、alox15b、ptgs2、ptgisPTGIS、atp1a2a(p<0.05)。其中txndc、alox15b、ptgs2、ptgis基因在黑化组中表达水平高于对照组;功能方面,有3个基因均与花生四烯酸(AA)有一定的相关性,这为多不饱和脂肪酸参与半滑舌鳎体色异常的分子调控机制提供了一定的理论支持。Abstract: Cynoglossus semilaevis as a representative species of Pleuronectiformes, is an important marine economic fish in Chinese coastal areas. The abnormal body color of C. semilaevis has been perplexing the practitioners for a long time. There are many reasons leading to abnormal body color, among which the genetic factors are thought to be the most fundamental reasons. The present researches on hypermelanosis of the blind side of C. semilaevis mainly focus on environment, nutrition, physiology, cloning of known pigmental genes and so on, while the digging of new funtional genes is still lacking. In this study, skin samples with different colors of C. semilaevis are selected for transcriptome sequencing. Through GO and KEGG functional enrichment and comparative analysis of different genes, differential expressed genes are screened in six melanogenesis-related KEGG pathways and the top ten genes are verified by qPCR. In this study, we find five functional genes with significant changes in the hypermelanotic skin on the blind side of C. semilaevis, which refer to txndc, alox15b, ptgs2, ptgis, and atp1a2a (p<0.05). The expression levels of txndc, alox15b, ptgs2, and ptgis genes in the melanization group are higher than those in the control group. In terms of function, three of these five genes are related to arachidonic acid (AA) to some extent. This provides theoretical support for the hypothesis that nutritional regulation related to unsaturated fatty acids may be involved in the molecular mechanism of abnormal body color in C. semilaevis.
-
Key words:
- skin /
- Cynoglossus semilaevis /
- hypermelanosis /
- arachidonic acid /
- transcriptome
-
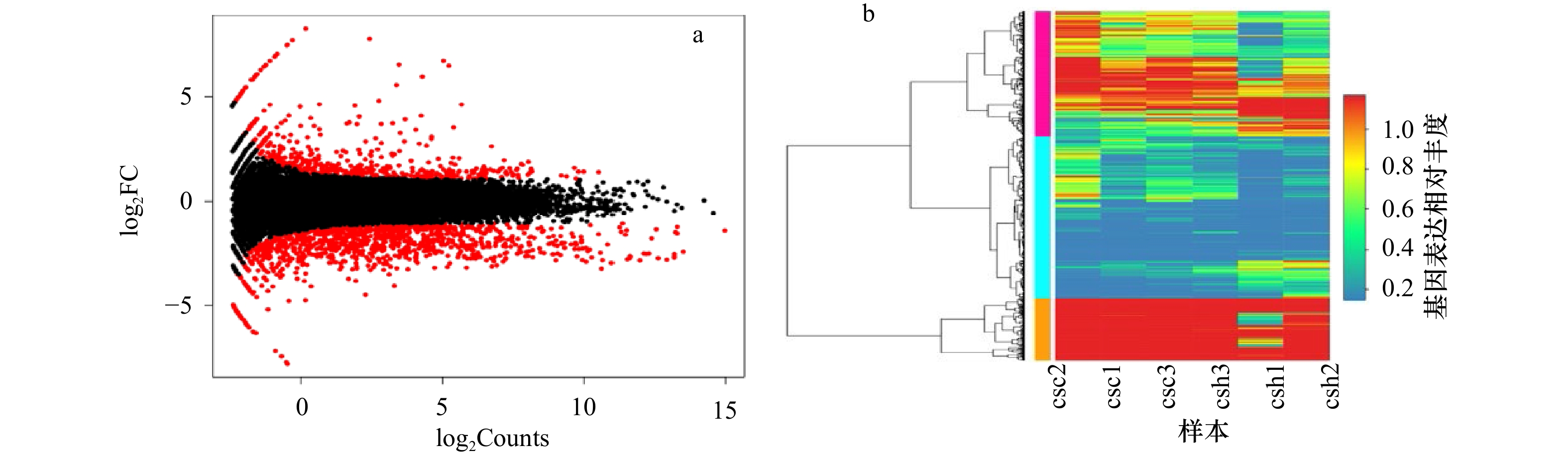
图 3 半滑舌鳎正常皮肤与黑化皮肤样品间差异表达基因火山图和差异表达基因丰度热图
a. 红色点代表差异基因,黑色点代表被过滤掉的数据,Count为测得计量数;b. 纵向代表基因类型
Fig. 3 Volcano map of the number of differentially expressed genes and heat map of abundance of differentially expressed gene between normal and hypermelanotic skin samples of Cynoglossus semilaevis
a. The red dots represent differential expressed genes, the black dots represent the filtered date, Counts is the measured quantity; b. the vertical one represents genes
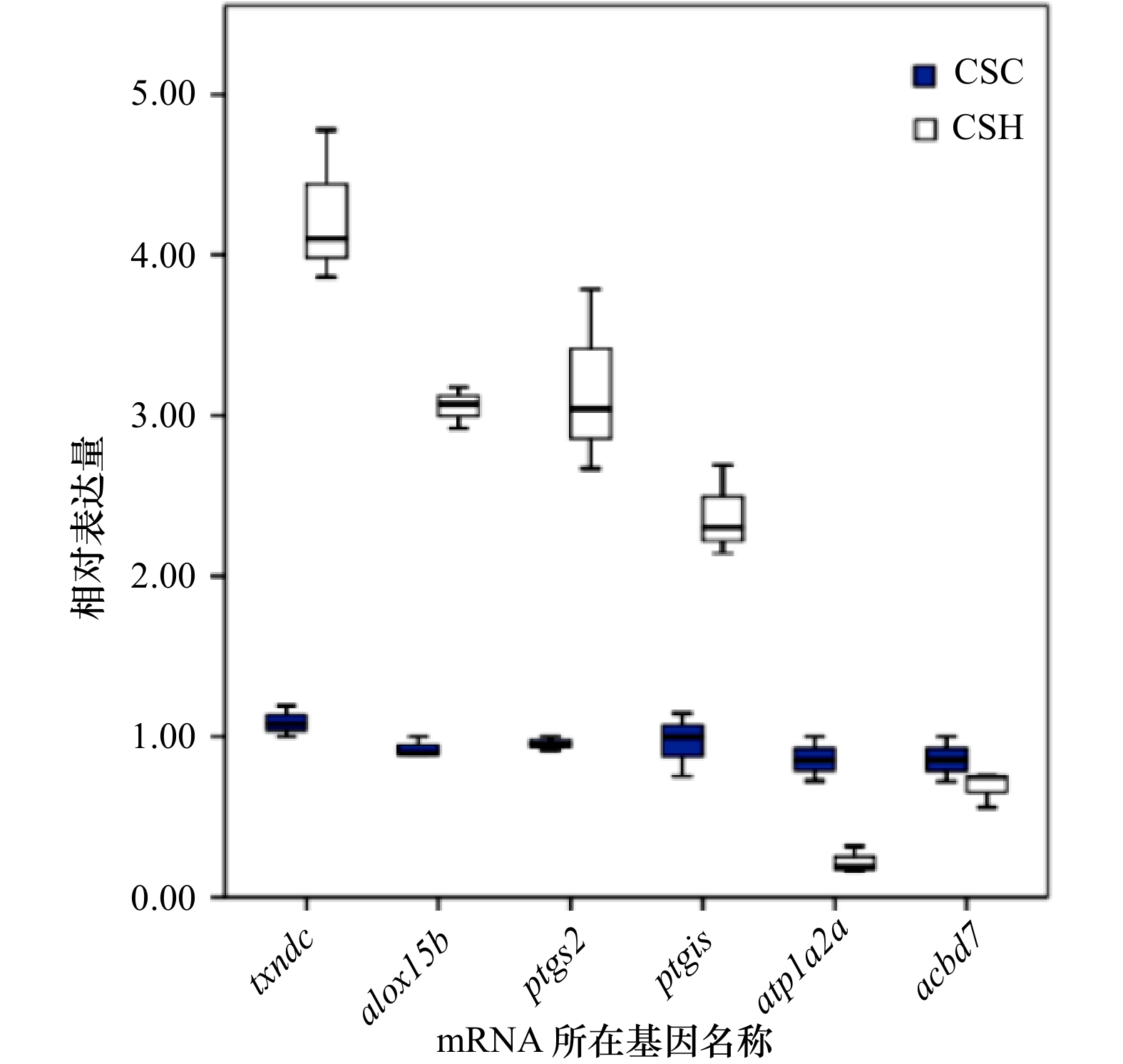
图 5 6个候选体色相关mRNA在半滑舌鳎6个测序样品中的qPCR表达检测结果箱线图
蓝色CSC代表半滑舌鳎皮肤正常对照组定量结果,白色CSH代表半滑舌鳎皮肤黑化实验组相对表达结果
Fig. 5 Box plot of qPCR expression results of 6 candidate body color-related mRNAs in 6 sequencing samples of Cynoglossus semilaevis
The CSC represents the relative expression in skin of normal C. semilaevis in the control group, and the CSH represents the relative expression in the skin of hypermelanotic C. semilaevis in the experimental group
表 1 6个半滑舌鳎皮肤转录组测序样品过滤后数据产出及质控分析
Tab. 1 Clean reads output and quality control analysis of six Cynoglossus semilaevis skin transcriptome sequencing samples
样本
名称总有效读数 碱基
数目Q20质量
分值Q30质量
分值GC含量
百分比/%N碱基
含量csc1 65 031 072 9 725 97.03 92.20 47.68 0 csc2 73 612 170 10 981 96.83 91.93 48.19 0 csc3 66 089 004 9 886 96.95 91.96 48.81 0 csh1 79 487 970 11 897 97.25 92.55 47.61 0 csh2 70 698 406 10 579 97.31 92.72 48.38 0 csh3 61 545 522 9 208 97.02 92.04 48.22 0 表 2 6个半滑舌鳎皮肤转录组测序样品基因组比对统计表
Tab. 2 Genomic comparison statistics of six Cynoglossus semilaevis skin transcriptome sequencing samples
样品名称 总数目 唯一比对到参考基因组上的
测得条目数目及占比比对到参考基因组多处的
测得条目数目及占比不能比对到参考基因组的
测得条目数目及占比csh1 39 743 985 32 335 924(81.36%) 1 897 171(4.77%) 5 510 890(13.87%) csc1 32 515 536 24 786 485(76.23%) 1 145 579(3.52%) 6 583 472(20.25%) csh2 35 349 203 28 571 030(80.83%) 1 563 099(4.42%) 5 215 074(14.75%) csc2 36 806 085 28 143 927(76.47%) 1 482 619(4.03%) 7 179 539(19.51%) csc3 33 044 502 25 549 970(77.32%) 1 369 439(4.14%) 6 125 093(18.54%) csh3 30 772 761 24 553 311(79.79%) 1 291 583(4.20%) 4 927 867(16.01%) -
[1] Odiorne J M. Degeneration of Melanophores in fundulus[J]. Proceedings of the National Academy of Sciences of the United States of America, 1933, 19(3): 329−332. doi: 10.1073/pnas.19.3.329 [2] Odiorne J M. Morphological color changes in vertebrates[J]. Annals of the New York Academy of Sciences, 1948, 4: 288−308. [3] Sugimoto M. Morphological color changes in fish: regulation of pigment cell density and morphology[J]. Microscopy Research & Technique, 2002, 58(6): 496−503. [4] 刘翠, 刘昊昆, 朱晓鸣, 等. 饲料中添加螺旋藻和叶黄素对杂交黄颡鱼生长、抗氧化能力和体色异常调控的比较研究[J]. 水生生物学报, 2021, 45(5): 1024−1033. doi: 10.7541/2021.2020.139Liu Cui, Liu Haokun, Zhu Xiaoming, et al. Comparative study on the regulation of growth, antioxidant capacity and abnormal body color of hybrid Pelteobagrus fulvidraco by adding Spirulina and lutein in feed[J]. Acta Hydrobiologica Sinica, 2021, 45(5): 1024−1033. doi: 10.7541/2021.2020.139 [5] Sugimoto M, Uchida N, Hatayama M. Apoptosis in skin pigment cells of the medaka, Oryzias latipes (Teleostei), during long-term chromatic adaptation: the role of sympathetic innervation[J]. Cell and Tissue Research, 2000, 301(2): 205−216. doi: 10.1007/s004410000226 [6] Hamre K, Holen E, Moren M. Pigmentation and eye migration in Atlantic halibut (Hippoglossus hippoglossus L. ) larvae: new findings and hypotheses[J]. Aquaculture Nutrition, 2007, 13(1): 65−80. doi: 10.1111/j.1365-2095.2007.00467.x [7] Youngson A F, Webb J H. Thyroid hormone levels in Atlantic salmon (Salmo salar) during the return migration from the ocean to spawn[J]. Journal of Fish Biology, 1993, 42(2): 293−300. doi: 10.1111/j.1095-8649.1993.tb00329.x [8] Vissio P G, Darias M J, Di Yorio M P, et al. Fish skin pigmentation in aquaculture: the influence of rearing conditions and its neuroendocrine regulation[J]. General and Comparative Endocrinology, 2021, 301: 113662. doi: 10.1016/j.ygcen.2020.113662 [9] 曹小龙. 斑马鱼报警物质和色素模式对其行为影响的研究[D]. 上海: 上海海洋大学, 2019.Cao Xiaolong. Effects of alarm substances and pigment patterns on behavior of zebrafish (Danio rerio)[D]. Shanghai: Shanghai Ocean University, 2019. [10] 邓成, 陈帅龙, 叶恒振, 等. 体色差异豹纹鳃棘鲈的色素及酶含量分析[J]. 生命科学研究, 2020, 24(1): 15−20.Deng Cheng, Chen Shuailong, Ye Hengzhen, et al. Analysis of pigment and enzyme levels of Plectropomus leopardus with body color difference[J]. Life Science Research, 2020, 24(1): 15−20. [11] 于道德, 张少春, 宋静静, 等. 鱼类色素细胞及其生态学意义概述[J]. 广西科学院学报, 2020, 36(2): 117−123.Yu Daode, Zhang Shaochun, Song Jingjing, et al. Overview of fish pigment cells and the ecological significance[J]. Journal of Guangxi Academy of Sciences, 2020, 36(2): 117−123. [12] 史学营, 徐永江, 武宁宁, 等. 半滑舌鳎(Cynoglossus semilaevis)体表色素细胞观察及POMC表达特性分析[J]. 渔业科学进展, 2015, 36(2): 45−54. doi: 10.11758/yykxjz.20150206Shi Xueying, Xu Yongjiang, Wu Ningning, et al. Preliminary studies on blind-side Hypermelanosis of Cynoglossus semilaevis: chromatophores observation and expression of proopiomelanocortin[J]. Progress in Fishery Sciences, 2015, 36(2): 45−54. doi: 10.11758/yykxjz.20150206 [13] 许细丹. 酪氨酸酶对瓯江彩鲤和斑马鱼黑斑体色影响的研究[D]. 上海: 上海海洋大学, 2020.Xu Xidan. Study on the effect of tyrosinase on black coloration in Oujiang color common carp and zebrafish[D]. Shanghai: Shanghai Ocean University, 2020. [14] 许璟瑾, 张文娟, 王静怡, 等. 金线莲抑制斑马鱼黑色素形成的活性组分筛选及机理研究[J]. 遗传, 2017, 39(12): 1178−1187.Xu Jingjin, Zhang Wenjuan, Wang Jingyi, et al. The active component screening of Anoectochilus roxburghii and the functional study on inhibition of melanogenesis in zebrafish[J]. Hereditas (Beijing), 2017, 39(12): 1178−1187. [15] 刘力, 裴思然, 吴华丽, 等. 基于tyrp1a转基因斑马鱼构建色素障碍性疾病药物筛选模型[J]. 中国药科大学学报, 2016, 47(6): 740−743.Liu Li, Pei Siran, Wu Huali, et al. Drug screening model of treating pigmentation disorders in tyrp1a transgenic zebrafish[J]. Journal of China Pharmaceutical University, 2016, 47(6): 740−743. [16] Lamason R L, Mohideen M P K, Mest J R, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans[J]. Science, 2005, 310(5755): 1782−1786. doi: 10.1126/science.1116238 [17] 赵美娟, 户晶晶, 倪辉, 等. 黑色素生成信号通路研究进展[J]. 生物工程学报, 2019, 35(9): 1633−1642.Zhao Meijuan, Hu Jingjing, Ni Hui, et al. Research progress in melanogenesis signaling pathway[J]. Chinese Journal of Biotechnology, 2019, 35(9): 1633−1642. [18] 芮孝. 鲤、鲫复制Sox10基因的表达和功能分化研究[D]. 舟山: 浙江海洋大学, 2018.Rui Xiao. Expression and functional differentiation of duplicated Sox10 genes in common carp and crucian carp[D]. Zhoushan: Zhejiang Ocean University, 2018. [19] 吴垚磊, 李仰真, 王娜, 等. 半滑舌鳎酪氨酸酶基因(TYR)和多巴色素异构酶基因(DCT)的克隆表达与分析[J]. 渔业科学进展, 2021, 42(6): 42−52.Wu Yaolei, Li Yangzhen, Wang Na, et al. Expression analysis of TYR and DCT genes related to body color in Cynoglossus semilaevis at different periods and in different tissues[J]. Progress in Fishery Sciences, 2021, 42(6): 42−52. [20] 傅建军, 朱文彬, 罗文韬, 等. 不同体色鲤的生长、酪氨酸酶活性、黑色素含量及基因表达比较[J]. 中国水产科学, 2021, 28(8): 919−947.Fu Jianjun, Zhu Wenbin, Luo Wentao, et al. Comparison of growth, tyrosinase activity, melanin content, and gene expression between common carps with different pigmentations[J]. Journal of Fishery Sciences of China, 2021, 28(8): 919−947. [21] 孟超, 徐皓, 黄超, 等. 鱼类体色相关功能基因的研究进展[J]. 湖南文理学院学报(自然科学版), 2020, 32(3): 30−35.Meng Chao, Xu Hao, Huang Chao, et al. Research progress on color-related genes of fish[J]. Journal of Hunan University of Arts and Science (Science and Technology), 2020, 32(3): 30−35. [22] Cal L, Suarez-Bregua P, Cerdá-Reverter J M, et al. Fish pigmentation and the melanocortin system[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2017, 211: 26−33. [23] Shelbourne J E. Population effects on the survival, growth and pigment of tank-reared plaice larvae[M]//Harden Jones F R. Sea Fisheries Research. London: Elsk, 1974: 729−735. [24] Seikai T, Matsumoto J. Mechanism of Pseudoalbinism in flatfish: an association between pigment cell and skin differentiation[J]. Journal of the World Aquaculture Society, 1994, 25(1): 78−85. [25] Tabata K. Application of the chromosomal manipulation in aquaculture of hirame Paralichthys olivaceus[J]. Bulletin of Hyogo Prefectural Fisheries Experimental Station, 1991, 28: 1−134. [26] Nakamura K, Iida H, Nakano H. Riboflavin in the skin of Albinic flatfish Liopsetta obscura[J]. Nippon Suisan Gakkaishi, 1986, 52(12): 2207. doi: 10.2331/suisan.52.2207 [27] Kanazawa A. Nutritional mechanisms involved in the occurrence of abnormal pigmentation in hatchery-reared flatfish[J]. Journal of the World Aquaculture Society, 1993, 24(2): 162−166. doi: 10.1111/j.1749-7345.1993.tb00005.x [28] Devresse B, Léger P, Sorgeloos P, et al. Improvement of flat fish pigmentation through the use of DHA-enriched rotifers and Artemia[J]. Aquaculture, 1994, 124(1/4): 287−288. [29] Shao Changwei, Bao Baolong, Xie Zhiyuan, et al. The genome and transcriptome of Japanese flounder provide insights into flatfish asymmetry[J]. Nature Genetics, 2017, 49(1): 119−124. doi: 10.1038/ng.3732 [30] Paterson E K, Ho H, Kapadia R, et al. 9-cis retinoic acid is the ALDH1A1 product that stimulates melanogenesis[J]. Experimental Dermatology, 2013, 22(3): 202−209. doi: 10.1111/exd.12099 [31] Takahashi A, Kosugi T, Kobayashi Y, et al. The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder[J]. General and Comparative Endocrinology, 2007, 151(2): 210−219. [32] 朱学武, 徐永江, 柳学周, 等. 池塘养殖牙鲆(Paralichthys olivaceus)无眼侧体色黑化消褪机理[J]. 渔业科学进展, 2017, 38(1): 103−110.Zhu Xuewu, Xu Yongjiang, Liu Xuezhou, et al. Physiological mechanisms for degeneration of blind-side Hypermelanosis in pond-cultured Japanese flounder (Paralichthys olivaceus)[J]. Progress in Fishery Sciences, 2017, 38(1): 103−110. [33] Koga A, Inagaki H, Bessho Y, et al. Insertion of a novel transposable element in the tyrosinase gene is responsible for an albino mutation in the medaka fish, Oryzias latipes[J]. Molecular and General Genetics MGG, 1995, 249: 400−405. doi: 10.1007/BF00287101 [34] Boonanuntanasarn S, Yoshizaki G, Iwai K, et al. Molecular cloning, gene expression in albino mutants and gene knockdown studies of tyrosinase mRNA in rainbow trout[J]. Pigment Cell Research, 2004, 17(4): 413−421. doi: 10.1111/j.1600-0749.2004.00166.x [35] Zhang X T, Weik J, Chen, Y Y, et al. Molecular cloning and expression analysis of tyr and tyrp1 genes in normal and albino yellow catfish Tachysurus fulvidraco[J]. Journal of Fish Biology, 2018, 92: 979−998. doi: 10.1111/jfb.13556 [36] 史学营, 柳学周, 石莹, 等. 半滑舌鳎 (Cynoglossus semilaevis)黑色素聚集素受体 (MCHR)表达特性及其与无眼侧黑化的关系[J]. 渔业科学进展, 2017, 38(1): 91−102.Shi Xueying, Liu Xuezhou, Shi Ying, et al. Molecular characterization of MCHR and its corelation with blind-side Hypermelanosis in Cynoglossus semilaevis[J]. Progress in Fishery Sciences, 2017, 38(1): 91−102. [37] 邱超达. SWS1介导的紫光/紫外光对斑马鱼皮肤黑色素细胞形成的影响[D]. 上海: 上海海洋大学, 2020.Qiu Chaoda. The influence of SWS1 mediated violet/ultra violet on the formation of cutaneous melanocyte cells in zebrafish[D]. Shanghai: Shanghai Ocean University, 2020. [38] Li Kunming, Zhao Na, Zhang Bo, et al. Identification and characterization of the melanocortin 1 receptor gene (MC1R) in hypermelanistic Chinese tongue sole (Cynoglossus semilaevis)[J]. Fish Physiology and Biochemistry, 2020, 46(3): 881−890. doi: 10.1007/s10695-019-00758-8 [39] Li Yangzhen, Hu Yuanri, Zheng Weiwei, et al. Insights into the heritable variation of hypermelanosis in Chinese tongue sole (Cynoglossus halflaevis): potential for future selective breeding[J]. Aquaculture, 2021, 539: 736617. doi: 10.1016/j.aquaculture.2021.736617 [40] 朱学武, 徐永江, 柳学周, 等. 半滑舌鳎黑色素聚集激素重组制备与生物活性分析[J]. 水产学报, 2016, 40(10): 1595−1605.Zhu Xuewu, Xu Yongjiang, Liu Xuezhou, et al. In vitro expression and bioactivity analysis of melanin concentration hormone from Cynoglossus semilaevis[J]. Journal of Fisheries of China, 2016, 40(10): 1595−1605. [41] 史学营. 养殖半滑舌鳎无眼侧黑化机制的初步研究[D]. 上海: 上海海洋大学, 2015.Shi Xueying. Preliminary investigation on mechanism for blind-side hyermelanosis of farmed tongue sole[D]. Shanghai: Shanghai Ocean University, 2015. [42] 马学坤, 柳学周, 温海深, 等. 半滑舌鳎早期发育过程中体表色素变化的研究[J]. 海洋水产研究, 2006, 27(2): 62−68.Ma Xuekun, Liu Xuezhou, Wen Haishen, et al. Changes of melanophores in the larval skin of Cynoglossus semilaevis Günther[J]. Marine Fisheries Research, 2006, 27(2): 62−68. [43] 朱学武. 养殖鲆鲽类无眼侧黑化调控机制研究[D]. 上海: 上海海洋大学, 2016.Zhu Xuewu. Studies on regulation mechanisms underline blind-side hypermelanosis of farmed flatfish[D]. Shanghai: Shanghai Ocean University, 2016. [44] Zhang Lin, Hou Yanhong, Li Nan, et al. The influence of TXNDC5 gene on gastric cancer cell[J]. Journal of Cancer Research and Clinical Oncology, 2010, 136(10): 1497−1505. doi: 10.1007/s00432-010-0807-x [45] Vincent E E, Elder D J E, Phillips L, et al. Overexpression of the TXNDC5 protein in non-small cell lung carcinoma[J]. Anticancer Research, 2011, 31(5): 1577−1582. [46] Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15)[J]. Gene, 2015, 573(1): 1−32. doi: 10.1016/j.gene.2015.07.073 [47] Kutzner L, Goloshchapova K, Heydeck D, et al. Mammalian ALOX15 orthologs exhibit pronounced dual positional specificity with docosahexaenoic acid[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2017, 1862(7): 666−675. [48] Sirois J, Sayasith K, Brown K A, et al. Cyclooxygenase-2 and its role in ovulation: a 2004 account[J]. Human Reproduction Update, 2004, 10(5): 373−385. doi: 10.1093/humupd/dmh032 [49] Shrestha K, Lukasik K, Baufeld A, et al. Regulation of ovulatory genes in bovine granulosa cells: lessons from siRNA silencing of PTGS2[J]. Reproduction, 2015, 149(1): 21−29. doi: 10.1530/REP-14-0337 [50] Lim H, Gupta R A, Ma Wenge, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ[J]. Genes & Development, 1999, 13(12): 1561−1574. [51] Nakayama T, Soma M, Izumi Y, et al. Organization of the human prostacyclin synthase gene[J]. Biochemical and Biophysical Research Communications, 1996, 221(3): 803−806. doi: 10.1006/bbrc.1996.0677 [52] 曾小芳. PTGIS基因突变对肺血管内皮细胞功能的影响[D]. 重庆: 重庆医科大学, 2018.Zeng Xiaofang. Effect of PTGIS gene mutation on the function of pulmonary vascular endothelial cells[D]. Chongqing: Chongqing Medical University, 2018. [53] De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2[J]. Nature Genetics, 2003, 33(2): 192−196. doi: 10.1038/ng1081 [54] Bruce M, Oyen F, Bell G, et al. Development of broodstock diets for the European Sea Bass (Dicentrarchus labrax) with special emphasis on the importance of n-3 and n-6 highly unsaturated fatty acid to reproductive performance[J]. Aquaculture, 1999, 177(1/4): 85−97. [55] Sargent J, McEvoy L, Estevez A, et al. Lipid nutrition of marine fish during early development: current status and future directions[J]. Aquaculture, 1999, 179(1/4): 217−229. [56] Van Anholt R D, Koven W M, Lutzky S, et al. Dietary supplementation with arachidonic acid alters the stress response of gilthead seabream (Sparus aurata) larvae[J]. Aquaculture, 2004, 238(1/4): 369−383. [57] Estévez A, McEvoy L A, Bell J G, et al. Growth, survival, lipid composition and pigmentation of turbot (Scophthalmus maximus) larvae fed live-prey enriched in Arachidonic and Eicosapentaenoic acids[J]. Aquaculture, 1999, 180(3/4): 321−343. [58] McEvoy L A, Estévez A, Bell J G, et al. Influence of dietary levels of eicosapentaenoic and arachidonic acids on the pigmentation success of turbot (Scophthalmus maximus L. ) and halibut (Hippoglossus hippoglossus L. )[J]. Bulletin of the Aquaculture Association of Canada, 1999(98-4): 17−20. [59] Copeman L A, Parrish C C, Brown J A, et al. Effects of docosahexaenoic, eicosapentaenoic, and arachidonic acids on the early growth, survival, lipid composition and pigmentation of yellowtail flounder (Limanda ferruginea): a live food enrichment experiment[J]. Aquaculture, 2002, 210(1/4): 285−304. [60] Bell J G, McEvoy L A, Estevez A, et al. Optimising lipid nutrition in first-feeding flatfish larvae[J]. Aquaculture, 2003, 227(1/4): 211−220. [61] Willey S, Bengtson D A, Harel M. Arachidonic acid requirements in larval summer flounder, Paralichthys dentatus[J]. Aquaculture International, 2003, 11(1): 131−149. [62] Villalta M, Estévez A, Bransden M P, et al. Arachidonic acid, arachidonic/eicosapentaenoic acid ratio, stearidonic acid and eicosanoids are involved in dietary-induced albinism in Senegal sole (Solea senegalensis)[J]. Aquaculture Nutrition, 2008, 14(2): 120−128. doi: 10.1111/j.1365-2095.2007.00511.x [63] Lund I, Steenfeldt S J, C B W. Effect of dietary arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on survival, growth and pigmentation in larvae of common sole (Solea solea L. )[J]. Aquaculture, 2007, 273(4): 532−544. doi: 10.1016/j.aquaculture.2007.10.047 [64] Boglino A, Wishkerman A, Darias M J, et al. The effects of dietary arachidonic acid on Senegalese sole morphogenesis: a synthesis of recent findings[J]. Aquaculture. 2014, 432: 443-452. [65] Ando H, Watabe H, Valencia J C, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function[J]. Journal of Biological Chemistry, 2004, 279(15): 15427−15433. doi: 10.1074/jbc.M313701200 [66] Estevez A, Kaneko T, Seikai T, et al. Ontogeny of ACTH and MSH cells in Japanese flounder (Paralichthys olivaceus) in relation to albinism[J]. Aquaculture, 2001, 202(1/2): 131−143. -




 下载:
下载: