Response of carbon sequestration capacity of Sargassum thunbergii to water flow speed
-
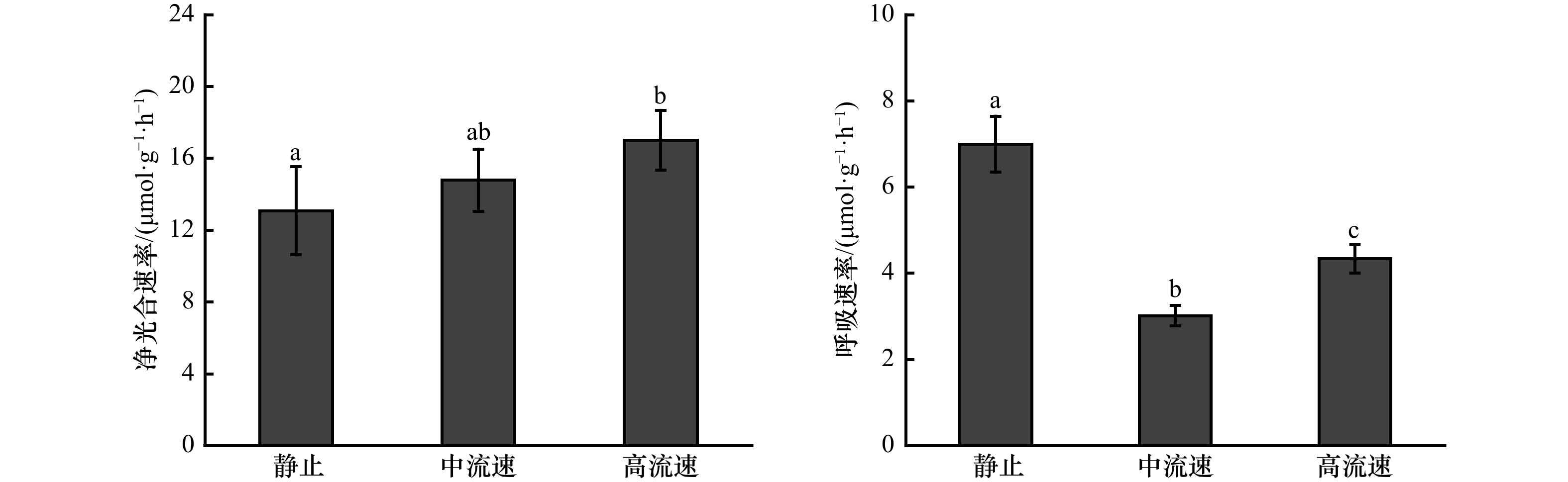
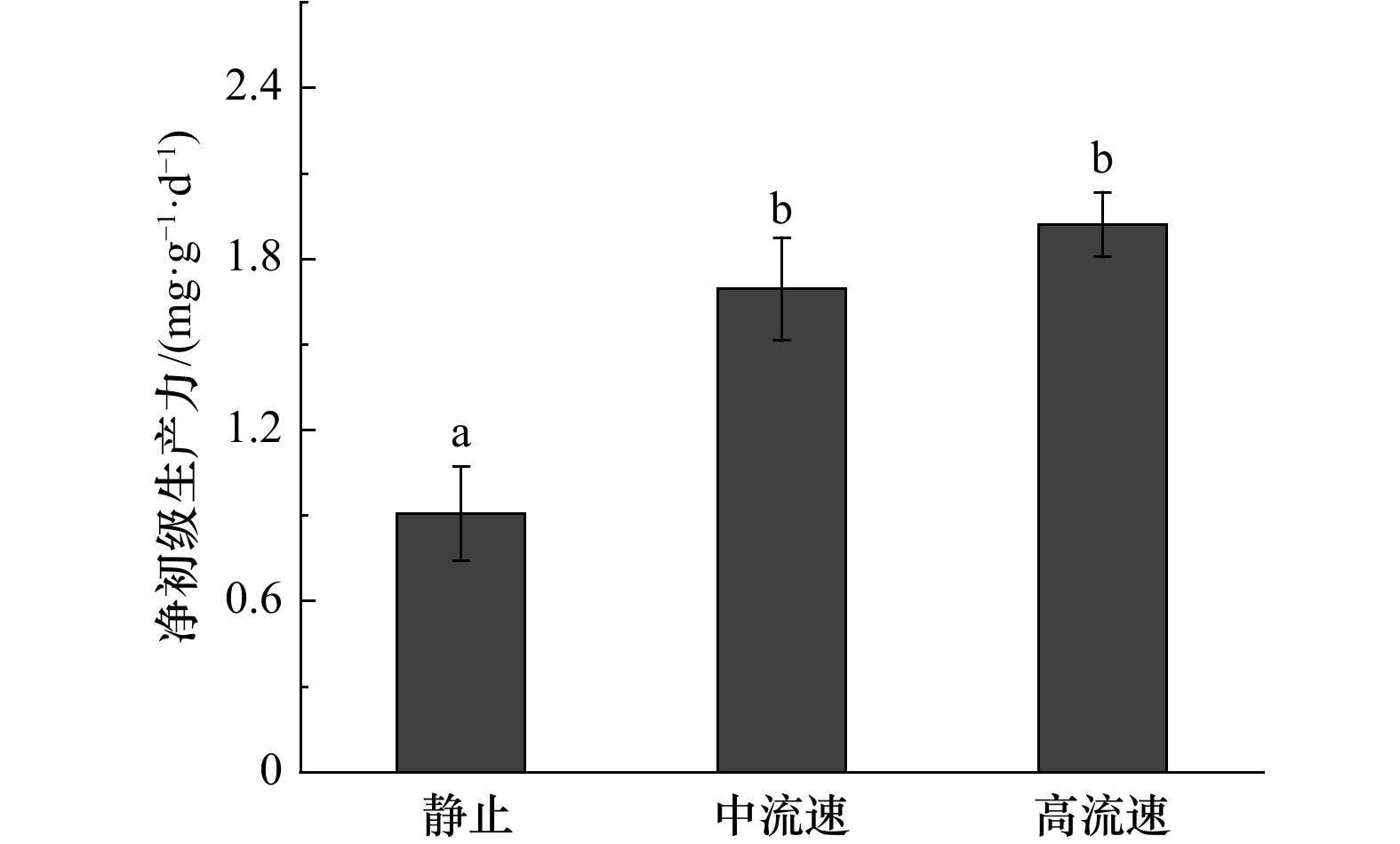
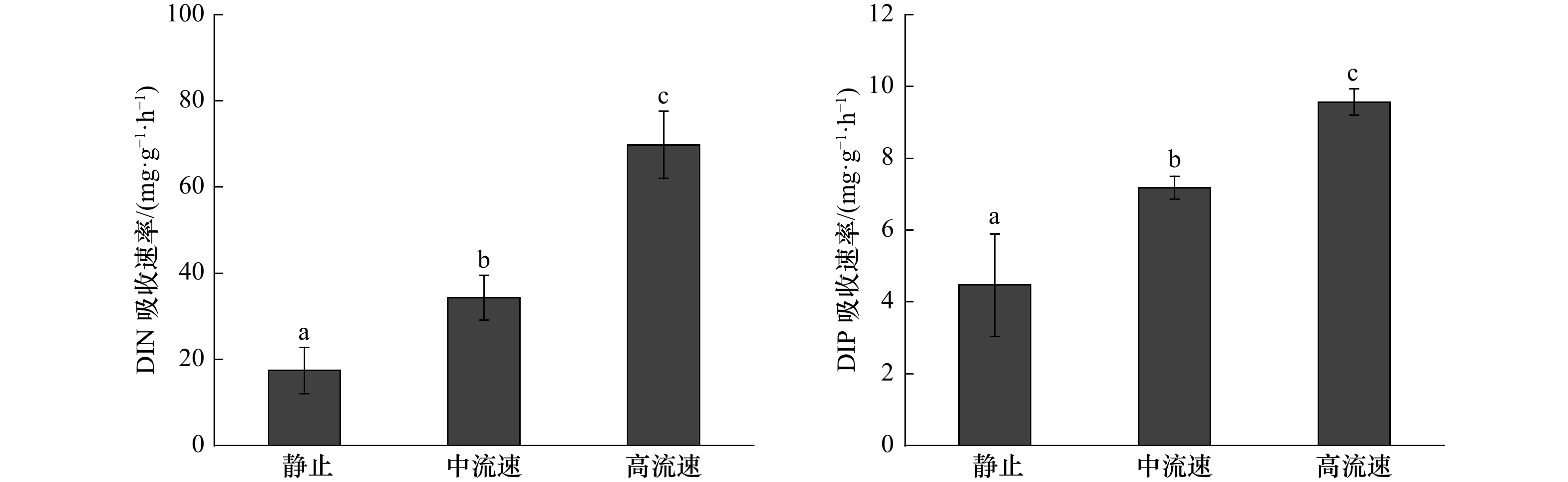
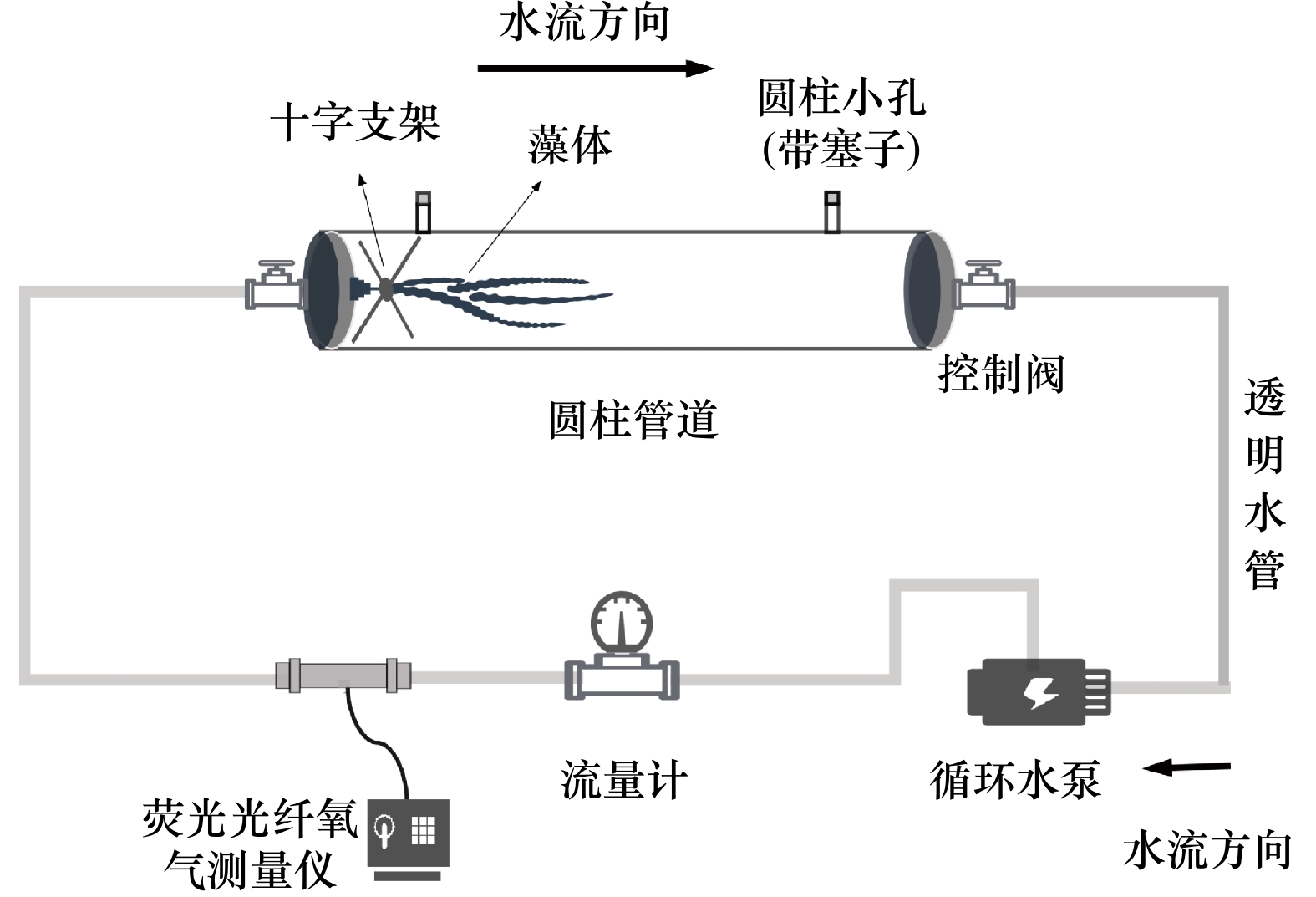
摘要: 流动的海水可以为海藻的生长提供所需的营养物质,对其生长和繁殖起到了非常重要的作用,而当前的生理生态学模拟实验,大多忽略了这一重要的环境因子。大型海藻虽然被认为是第4类“蓝碳”,但关于其固碳能力的研究较少。本研究设计了一种可以调节流速的大型海藻固碳能力测量系统,既可以测量大型海藻的净光合速率、呼吸速率和对无机氮、无机磷的吸收速率,也可以测量可溶性有机碳的释放速率。结果显示,与静止(0 m/s)相比,中流速(0.033 m/s)和高流速(0.094 m/s)均能提高鼠尾藻的净光合速率、净初级生产力、可溶性有机碳释放速率以及对无机氮、无机磷的吸收速率,且最大值均出现在高流速下。此外,鼠尾藻的可溶性有机碳释放速率随净初级生产力的提高而提高。本测量系统可为大型海藻固碳能力的研究提供切实可行的参考。Abstract: The flowing seawater can provide the nutrients for the growth of marine plants, which plays a vital role in the growth and reproduction of marine plants. However, most of the current simulation physiological and ecological experiments ignored this important environmental factor. Although macroalgae is regarded as the emerging fourth category of “blue carbon”, there are few studies on its carbon sequestration. In this study, we design a carbon sequestration capacity measurement system of macroalgae that can adjust the flow speed, which can not only measure the photosynthetic rate, respiratory rate and the absorption rate of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP), but also measure the release rate of dissolved organic carbon (DOC) of macroalgae. The results show that the net photosynthetic rate, net primary productivity, DOC release rate and the absorption rate of DIN and DIP of Sargassum thunbergii are increased at medium (0.033 m/s) and high (0.094 m/s) flow speed compared with static status (0 m/s), and the highest values are obtained under high flow speed (0.094 m/s). In addition, the DOC release rate of S. thunbergii increased with the increase of the net primary productivity. This measurement system can provide a practical reference for the study of carbon sequestration capacity of macroalgae.
-
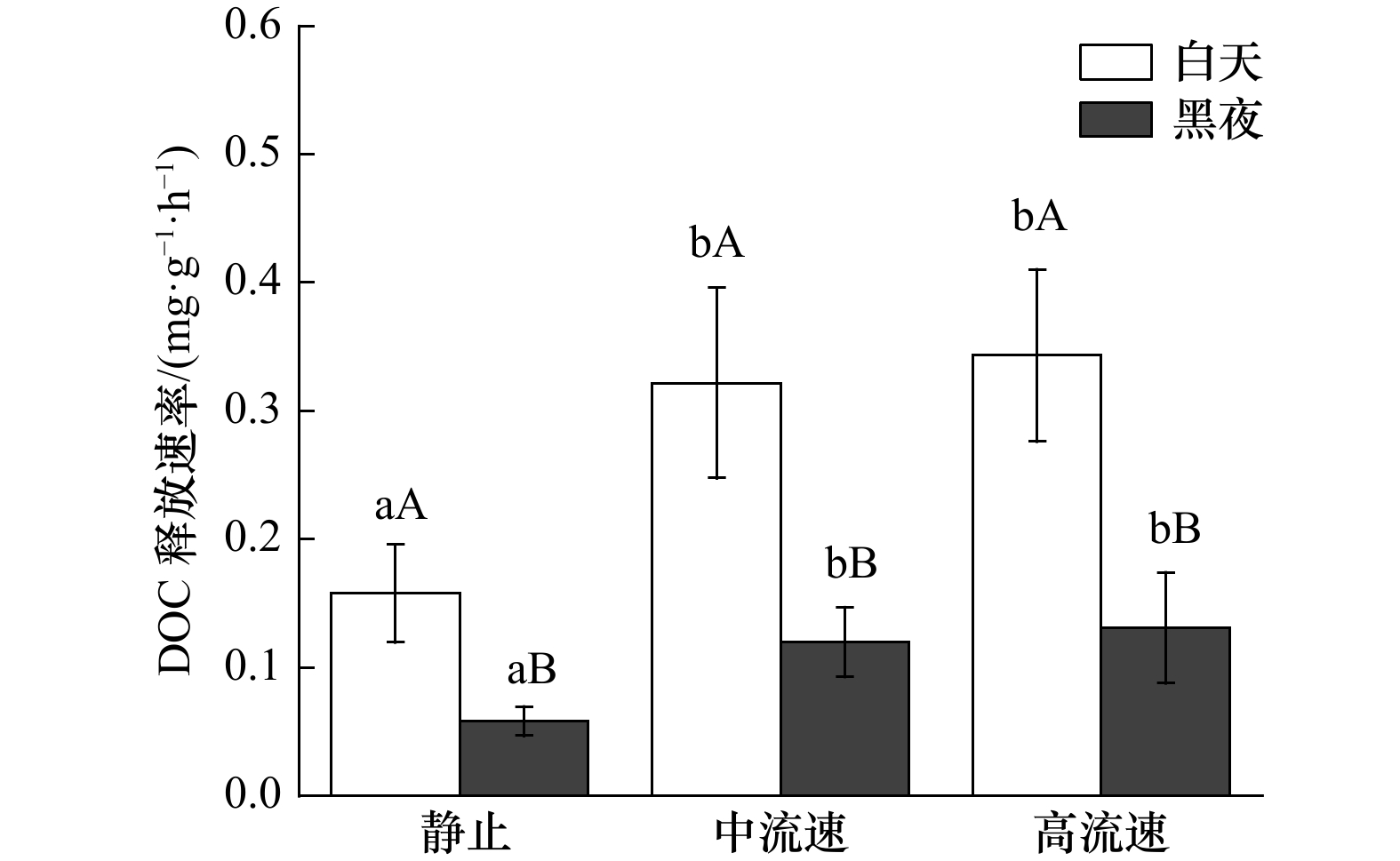
图 6 不同流速下鼠尾藻的可溶性有机碳(DOC)释放速率
不同的小写字母表示不同流速下,DOC释放速率的显著性差异(p<0.05);不同的大写字母表示相同流速下,白天和黑夜DOC释放速率的显著性差异(p<0.05)
Fig. 6 The dissolved organic carbon (DOC) release rate of Sargassum thunbergii under different flow rates
Different lowercase letters indicate a significant difference in DOC release rate at different flow rates (p<0.05); different capital letters indicate a significant difference in DOC release rate between day and night at the same flow rate (p<0.05)
表 1 鼠尾藻在中流速培养24 h的各项有机碳量
Tab. 1 The organic carbon content of Sargassum thunbergii cultured at a medium flow rate for 24 h
装置编号 C净/mg POC1/mg POC2/mg DOC/mg (POC1+POC2+DOC)/mg C差/mg C差/C净 A 48.78 12.86 22.20 12.70 47.76 1.01 2.08% B 47.16 10.92 23.55 11.80 46.27 0.89 1.88% C 63.91 16.14 29.58 16.80 62.52 1.39 2.18% 平均值±标准差 53.28±9.24 13.31±2.64 25.11±3.93 13.77±2.67 52.19±8.98 1.10±0.26 (2.05±0.15)% 注:装置A、B和C是实验的3个重复组;C净:24 h的净固碳量;POC1:24 h藻体增加的生物量和枯枝败叶中的有机碳量;POC2:24 h培养水体中的颗粒有机碳量;DOC:24 h藻体释放的溶解有机碳量;C差=C净−(POC1+POC2+DOC)。 -
[1] 范振林. 开发蓝色碳汇助力实现碳中和[J]. 中国国土资源经济, 2021, 34(4): 12−18.Fan Zhenlin. Developing blue carbon sink to implement carbon neutralization[J]. Natural Resource Economics of China, 2021, 34(4): 12−18. [2] Devries T. The oceanic anthropogenic CO2 sink: storage, air-sea fluxes, and transports over the industrial era[J]. Global Biogeochemical Cycles, 2014, 28(7): 631−647. doi: 10.1002/2013GB004739 [3] DeVries T, Holzer M, Primeau F. Recent increase in oceanic carbon uptake driven by weaker upper-ocean overturning[J]. Nature, 2017, 542(7640): 215−218. doi: 10.1038/nature21068 [4] Behrenfeld M J, Marañón E, Siegel D A, et al. Photoacclimation and nutrient-based model of light-saturated photosynthesis for quantifying oceanic primary production[J]. Marine Ecology Progress Series, 2002, 228: 103−117. doi: 10.3354/meps228103 [5] Homén K. The global carbon cycle[J]. International Geophysics, 2000, 72: 282−321. [6] Duarte C M, Middelburg J J, Caraco N. Major role of marine vegetation on the oceanic carbon cycle[J]. Biogeosciences, 2005, 2(1): 1−8. doi: 10.5194/bg-2-1-2005 [7] Alpert S B, Spencer D F, Hidy G. Biospheric options for mitigating atmospheric carbon dioxide levels[J]. Energy Conversion and Management, 1992, 33(5/8): 729−736. [8] Duarte C M, Cebrián J. The fate of marine autotrophic production[J]. Limnology and Oceanography, 1996, 41(8): 1758−1766. doi: 10.4319/lo.1996.41.8.1758 [9] Krause-Jensen D, Duarte C M. Substantial role of macroalgae in marine carbon sequestration[J]. Nature Geoscience, 2016, 9(10): 737−742. doi: 10.1038/ngeo2790 [10] Han T, Han Y S, Kain J M, et al. Thallus differentiation of photosynthesis, growth, reproduction, and UV-B sensitivity in the green alga Ulva pertusa (Chlorophyceae)[J]. Journal of Phycology, 2003, 39(4): 712−721. doi: 10.1046/j.1529-8817.2003.02155.x [11] Wu Hailong, Feng Jingchi, Li Xinshu, et al. Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China[J]. Marine Pollution Bulletin, 2019, 146: 639−644. doi: 10.1016/j.marpolbul.2019.07.025 [12] Gao K, Umezaki I. Comparative photosynthetic capacities of the leaves of upper and lower parts of sargassum plants[J]. Botanica Marina, 1988, 31(3): 231−236. [13] Horner S M J, Smith D F. Distinguishing modes of dissolved organic carbon production in marine macrophytes by tracer kinetic analysis[J]. Marine Biology, 1984, 81(3): 231−236. doi: 10.1007/BF00393217 [14] Schumacher G J, Whitford L A. Respiration and P32 uptake in various species of freshwater algae as affected by a current[J]. Journal of Phycology, 1965, 1(2): 78−80. doi: 10.1111/j.1529-8817.1965.tb04561.x [15] Carpenter R C. Relationships between primary production and irradiance in coral reef algal communities[J]. Limnology and Oceanography, 1985, 30(4): 784−793. doi: 10.4319/lo.1985.30.4.0784 [16] Griffith P C, Cubit J D, Adey W H, et al. Computer-automated flow respirometry: metabolism measurements on a Caribbean reef flat and in a microcosm[J]. Limnology and Oceanography, 1987, 32(2): 442−451. doi: 10.4319/lo.1987.32.2.0442 [17] Cornwall C E, Hepburn C D, Pilditch C A, et al. Concentration boundary layers around complex assemblages of macroalgae: implications for the effects of ocean acidification on understory coralline algae[J]. Limnology and Oceanography, 2013, 58(1): 121−130. doi: 10.4319/lo.2013.58.1.0121 [18] Nishihara G N, Terada R. Spatial variations in nutrient supply to the red algae Eucheuma serra (J. Agardh) J. Agardh[J]. Phycological Research, 2010, 58(1): 29−34. doi: 10.1111/j.1440-1835.2009.00555.x [19] Wingler A, Lea P J, Quick W P, Leegood R C. Photorespiration: metabolic pathways and their role in stress protection[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2000, 355(1402): 1517−1529. doi: 10.1098/rstb.2000.0712 [20] Weigel B L, Pfister C A. The dynamics and stoichiometry of dissolved organic carbon release by kelp[J]. Ecology, 2021, 102(2): e03221. [21] 高坤山. 藻类光合固碳的研究技术与解析方法[J]. 海洋科学, 1999(6): 37−41. doi: 10.3969/j.issn.1000-3096.1999.06.017Gao Kunshan. Research techniques and methods in characterizing photosynthetic carbon fixation by algae[J]. Marine Sciences, 1999(6): 37−41. doi: 10.3969/j.issn.1000-3096.1999.06.017 [22] Bao Menglin, Hu Lili, Fu Qianqian, et al. Different photosynthetic responses of Pyropia yezoensis to ultraviolet radiation under changing temperature and photosynthetic active radiation regimes[J]. Photochemistry and Photobiology, 2019, 95(5): 1213−1218. doi: 10.1111/php.13108 [23] Liu Fuli, Sun Xiutao, Wang Wenjun, et al. Development of a female-specific RAPD marker for Sargassum thunbergii gender identification using bulked segregant analysis[J]. Aquatic Botany, 2012, 102: 79−81. doi: 10.1016/j.aquabot.2012.05.001 [24] 刘玮, 辛美丽, 吕芳, 等. 关于鼠尾藻群体数量分布的三种统计模型比较[J]. 生态学报, 2018, 38(6): 2031−2040.Liu Wei, Xin Meili, Lü Fang, et al. Comparison of three statistical models for the quantitative distribution of Sargassum thunbergii populations[J]. Acta Ecologica Sinica, 2018, 38(6): 2031−2040. [25] Zhang Quansheng, Li Wei, Liu Su, et al. Size-dependence of reproductive allocation of Sargassum thunbergii (Sargassaceae, Phaeophyta) in Bohai Bay, China[J]. Aquatic Botany, 2009, 91(3): 194−198. doi: 10.1016/j.aquabot.2009.06.003 [26] 于永强, 张全胜, 唐永政, 等. 马尾藻种群生活史性状时空变化的研究进展[J]. 水产科学, 2012, 31(7): 437−443. doi: 10.3969/j.issn.1003-1111.2012.07.011Yu Yongqiang, Zhang Quansheng, Tang Yongzheng, et al. The advance in research on spatio-temporal variation in life cycle in sea weed Sargassum[J]. Fisheries Science, 2012, 31(7): 437−443. doi: 10.3969/j.issn.1003-1111.2012.07.011 [27] Wada S, Aoki M N, Tsuchiya Y, et al. Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan[J]. Journal of Experimental Marine Biology and Ecology, 2007, 349(2): 344−358. doi: 10.1016/j.jembe.2007.05.024 [28] Gao Kunshan, Xu Juntian, Zheng Yangqiao, et al. Measurement of benthic photosynthesis and calcification in flowing-through seawater with stable carbonate chemistry[J]. Limnology and Oceanography: Methods, 2012, 10(7): 555−559. doi: 10.4319/lom.2012.10.555 [29] Cory R M, Harrold K H, Neilson B T, et al. Controls on dissolved organic matter (DOM) degradation in a headwater stream: the influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation[J]. Biogeosciences, 2015, 12(22): 6669−6685. doi: 10.5194/bg-12-6669-2015 [30] Mopper K, Zhou Xianliang, Kieber R J, et al. Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle[J]. Nature, 1991, 353(6339): 60−62. doi: 10.1038/353060a0 [31] Carpenter R C, Hackney J M, Adey W H. Measurements of primary productivity and nitrogenase activity of coral reef algae in a chamber incorporating oscillatory flow[J]. Limnology and Oceanography, 1991, 36(1): 40−49. doi: 10.4319/lo.1991.36.1.0040 [32] Koehl M A R, Alberte R S. Flow, flapping, and photosynthesis of Nereocystis leutkeana: a functional comparison of undulate and flat blade morphologies[J]. Marine Biology, 1988, 99(3): 435−444. doi: 10.1007/BF02112137 [33] Ghisalberti M, Nepf H M. Mixing layers and coherent structures in vegetated aquatic flows[J]. Journal of Geophysical Research: Oceans, 2002, 107(C2): 3011. doi: 10.1029/2001JC000871 [34] Wheeler W N. Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera[J]. Marine Biology, 1980, 56(2): 103−110. doi: 10.1007/BF00397128 [35] Koch E W. Hydrodynamics, diffusion-boundary layers and photosynthesis of the seagrasses Thalassia testudinum and Cymodocea nodosa[J]. Marine Biology, 1994, 118(4): 767−776. doi: 10.1007/BF00347527 [36] Gao K S. Effects of seawater current speed on the photosynthetic oxygen evolution of Sargassum thunbergii (Phaeophyta)[J]. Japanese Journal of Phycology, 1991, 39: 291−293. [37] Wyatt K H, Rober A R, Schmidt N, et al. Effects of desiccation and rewetting on the release and decomposition of dissolved organic carbon from benthic macroalgae[J]. Freshwater Biology, 2014, 59(2): 407−416. doi: 10.1111/fwb.12273 [38] Fogg G E, Nalewajko C, Watt W D. Extracellular products of phytoplankton photosynthesis[J]. Proceedings of the Royal Society of London. Series B, Biological Sciences, 1965, 162(989): 517−534. [39] Morán X A G, Estrada M. Phytoplanktonic DOC and POC production in the Bransfield and Gerlache Straits as derived from kinetic experiments of 14C incorporation[J]. Deep-Sea Research Part II: Topical Studies in Oceanography, 2002, 49(4/5): 769−786. [40] Aluwihare L I, Repeta D J, Chen R F. A major biopolymeric component to dissolved organic carbon in surface sea water[J]. Nature, 1997, 387(6629): 166−169. doi: 10.1038/387166a0 [41] 殷建平, 王友绍, 徐继荣, 等. 海洋碳循环研究进展[J]. 生态学报, 2006, 26(2): 566−575. doi: 10.3321/j.issn:1000-0933.2006.02.033Yin Jianping, Wang Youshao, Xu Jirong, et al. Adavances of studies on marine carbon cycle[J]. Acta Ecologica Sinica, 2006, 26(2): 566−575. doi: 10.3321/j.issn:1000-0933.2006.02.033 [42] Carlson C A, Ducklow H W. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea[J]. Aquatic Microbial Ecology, 1996, 10(1): 69−85. [43] Hill R, Bellgrove A, Macreadie P I, et al. Can macroalgae contribute to blue carbon? An Australian perspective[J]. Limnology and Oceanography, 2015, 60(5): 1689−1706. doi: 10.1002/lno.10128 [44] Marañón E, Cermeño P, Fernández E, et al. Significance and mechanisms of photosynthetic production of dissolved organic carbon in a coastal eutrophic ecosystem[J]. Limnology and Oceanography, 2004, 49(5): 1652−1666. doi: 10.4319/lo.2004.49.5.1652 [45] Bjørrisen P K. Phytoplankton exudation of organic matter: why do healthy cells do it?[J]. Limnology and Oceanography, 1988, 33(1): 151−154. doi: 10.4319/lo.1988.33.1.0151 [46] Berman T. Release of dissolved organic matter by photosynthesizing algae in Lake Kinneret, Israel[J]. Freshwater Biology, 1976, 6(1): 13−18. doi: 10.1111/j.1365-2427.1976.tb01585.x [47] Abdullah M I, Fredriksen S. Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway[J]. Journal of the Marine Biological Association of the United Kingdom, 2004, 84(5): 887−894. doi: 10.1017/S002531540401015Xh [48] Reed D C, Carlson C A, Halewood E R, et al. Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera[J]. Limnology and Oceanography, 2015, 60(6): 1996−2008. doi: 10.1002/lno.10154 [49] 张林海, 曾从盛, 胡伟芳. 氮输入对植物光合固碳的影响研究进展[J]. 生态学报, 2017, 37(1): 147−155.Zhang Linhai, Zeng Congsheng, Hu Weifang. Reviews on effects of nitrogen addition on plant photosynthetic carbon fixation[J]. Acta Ecologica Sinica, 2017, 37(1): 147−155. [50] Juneja A, Ceballos R M, Murthy G S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review[J]. Energies, 2013, 6(9): 4607−4638. doi: 10.3390/en6094607 [51] Cornelisen C D, Thomas F I M. Water flow enhances ammonium and nitrate uptake in a seagrass community[J]. Marine Ecology Progress Series, 2006, 312: 1−13. doi: 10.3354/meps312001 [52] Gerard V A. In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera[J]. Marine Biology, 1982, 69(1): 51−54. doi: 10.1007/BF00396960 [53] Touchette B W, Burkholder J M. Review of nitrogen and phosphorus metabolism in seagrasses[J]. Journal of Experimental Marine Biology and Ecology, 2000, 250(1/2): 133−167. [54] Hurd C L, Harrison P J, Druehl L D. Effect of seawater velocity on inorganic nitrogen uptake by morphologically distinct forms of Macrocystis integrifolia from wave-sheltered and exposed sites[J]. Marine Biology, 1996, 126(2): 205−214. doi: 10.1007/BF00347445 [55] Thomas F I M, Cornelisen C D. Ammonium uptake by seagrass communities: effects of oscillatory versus unidirectional flow[J]. Marine Ecology Progress Series, 2003, 247: 51−57. doi: 10.3354/meps247051 [56] Atkinson M J, Smith S V. C∶N∶P ratios of benthic marine plants[J]. Limnology and Oceanography, 1983, 28(3): 568−574. doi: 10.4319/lo.1983.28.3.0568 [57] Falkowski P G. Rationalizing elemental ratios in unicellular algae[J]. Journal of Phycology, 2000, 36(1): 3−6. doi: 10.1046/j.1529-8817.2000.99161.x -




 下载:
下载: