Histological observation on gonadal differentiation and first annual gonadal development of cobia (Rachycentron canadum)
-
摘要: 本研究采用石蜡组织切片和H.E.染色法对军曹鱼原始性腺的形成、分化及精巢和卵巢首周年发育的组织结构变化进行观察。结果显示,军曹鱼原始生殖细胞在7孵化日龄(days post hatching,dph)时迁移到达生殖嵴,随后体细胞出现聚集和分裂,并于15 dph将原始生殖细胞完全包绕形成原始性腺。军曹鱼卵巢分化的时间要早于精巢,34 dph的稚鱼性腺组织尚未分化,但可观察到两种存在明显组织学差异的性腺类型,其中一种类型的性腺出现卵原细胞群,而另一种类型的性腺横切面较狭长,生殖细胞数量明显较少,因此推定为未分化精巢;卵巢的解剖学分化开始于44 dph,其标志为卵巢腔的形成;50 dph时精巢开始细胞学上的分化,此时精原细胞由基底膜包被形成囊泡状的细胞团。在首周年发育期间 (60~360 dph),军曹鱼的精巢发育包含I、II、III、IV、V 5个时期,而卵巢发育只包含I、II、III 3个时期。60 dph时,精巢和卵巢均处于I期;90 dph时,精巢发育至II期,卵巢仍处于I期;120 dph时,超过半数的精巢已发育至 III期,仅少部分卵巢发育至II期;150 dph时,精巢已发育至III期,而大部分卵巢发育至II期;185 dph时,精巢仍为III期,卵巢均已发育至II期;210 dph时,大部分精巢发育至IV期,卵巢仍处于II期;360 dph时,精巢已发育至V期,大部分卵巢发育至III期。上述研究结果可丰富军曹鱼的繁殖生物学研究基础,阐明其早期性腺发育规律,还可为其人工繁殖提供理论依据。Abstract: In this study, histological observation of gonadogenesis, gonadal differentiation and first annual gonadal development in cobia (Rachycentron canadum) was performed based on paraffin section and H.E. staining method. The results of histological observations showed that the primordial germ cells (PGCs) arrived at the genital ridge at 7 dph (days post hatching), then the somatic cells began to aggregate around the PGCs. At 15 dph, the PGCs were enclosed entirely by somatic cells and formed the primary gonad. Two types of primary gonads with noticeable histological differences could be observed at 34 dph. The presence of clusters of oogonia characterized a part of primary gonads. The other primary gonads showed a narrower cross-section with fewer germ cells, which were presumed to be undifferentiated testis. Similar to other fishes, ovarian differentiation of cobia was anterior to testicular differentiation. The anatomical indication of ovarian differentiation was the ovarian cavity formation, which occurred at 44 dph. The cytological differentiation of testis began at about 50 dph and was characterized by the presence of spermatogonial acinar-clusters. The first annual development of testes could be divided into five stages (I, II, III, IV, V), while the development of the ovaries was divided into three stages (I, II, III). Testes and ovaries were both developed to early Stage I at 60 dph. All of the testes developed to Stage II at 90 dph; the ovaries were still at Stage I. More than half of the testes developed to Stage III and part of the ovaries developed to Stage II at 120 dph. The testes were mainly developed to Stage III at 150 dph; most of the ovaries were at Stage II. The testes were still at stage III, and the ovaries were developed to Stage II at 185 dph. Most of the testes developed to Stage IV at 210 dph, but the ovaries were still at Stage II. At 360 dph, the testes entered Stage V, and most of the ovaries developed to Stage III. These findings could clarify gonadal development characteristics and provide a theoretical basis for the artificial breeding of cobia.
-
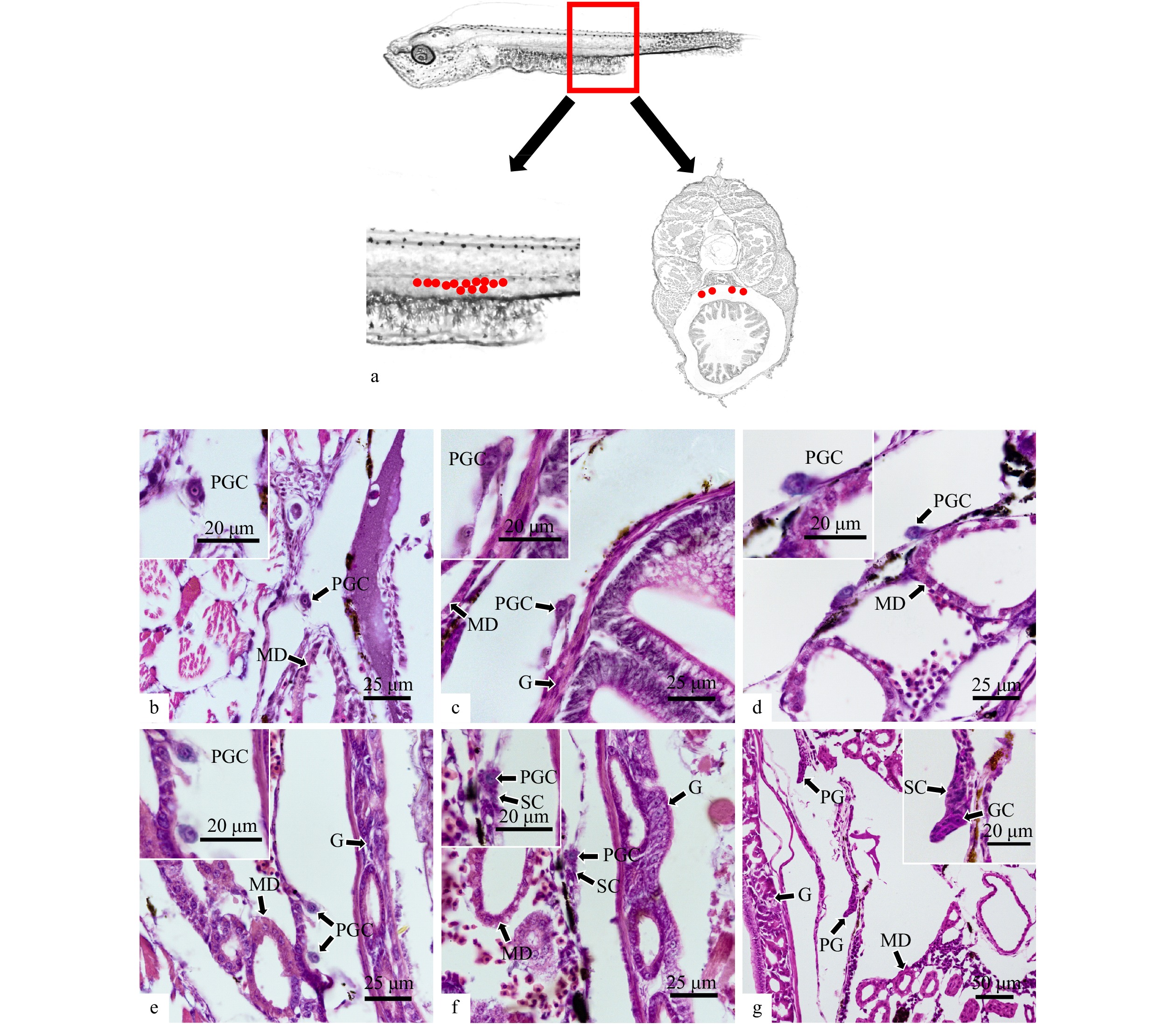
图 1 军曹鱼原始生殖细胞的胚后迁移
a. 原始生殖细胞分布位置示意图(红点表示原始生殖细胞);b, c. 3 dph和5 dph单个游离存在的原始生殖细胞;d, e. 7 dph和9 dph原始生殖细胞迁移至生殖嵴;f. 11 dph原始生殖细胞周围开始出现体细胞聚集;g. 15 dph原始生殖细胞被体细胞包围形成原始性腺雏形;PGC. 原始生殖细胞;GC. 生殖细胞;SC. 体细胞;PG. 原始性腺;MD. 中肾管;G. 肠
Fig. 1 Migration of primordial germ cells of Rachycentron canadum during early development
a. Schematic diagram of PGCs distribution (red dots indicated primordial germ cells); b, c. individual PGC at 3 dph and 5 dph; d, e. PGCs arrive at the genital ridge at 7 dph and 9 dph; f. somatic cells began to aggregate around PGCs at 11 dph; g. somatic cells encompassed PGCs and the prototype primary gonad was formed at 15 dph; PGC. primordial germ cells; GC. germ cell; SC. somatic cell; PG. primary gonad; MD. mesonephric duct; G. gut
图 2 军曹鱼原始性腺的分化
a−e. 18 dph、21 dph、24 dph、27 dph和30 dph尚未出现分化特征的原始性腺;f. 34 dph早期卵巢,形成卵原细胞群;g. 34 dph推定的精巢组织;h. 40 dph早期精巢,精原细胞出现;i. 50 dph早期精巢,形成精原细胞囊;j. 44 dph早期卵巢,出现卵巢腔;k. 48 dph早期卵巢,卵巢腔扩大;l. 52 dph早期卵巢,卵原细胞开始向初级卵母细胞过渡;BC. 血细胞;BV. 血管;GC. 生殖细胞;PG. 原始性腺;O. 卵巢;OG. 卵原细胞;PT. 推定的精巢组织;T. 精巢;SG. 精原细胞
Fig. 2 Differentiation of primary gonad of Rachycentron canadum
a−e. Undifferentiated primary gonad at 18 dph, 21 dph, 24 dph, 27 dph and 30 dph; f. early differentiated ovary at 34 dph, clusters of oogonia formed; g. presumptive testis at 34 dph; h. early differentiated testis at 40 dph, clusters of spermatogonia formed; i. early differentiated testis at 50 dph, cysts of spermatogonia formed; j. early differentiated ovary at 44 dph, ovarian cavity formed; k. early differentiated ovary at 48 dph, ovarian cavity increased in size; l. early differentiated ovary at 52 dph, oogonia began to transform into primary oocytes; BC. blood cell; BV. blood vessel; GC. germ cell; PG. primary gonad; O. ovary; OG. oogonia; PT. presumptive testis; T. testis; SG: spermatogonia
图 3 军曹鱼精巢首周年发育的组织学变化
A, a. 60 dph精巢切片;B, b. 90 dph 精巢切片;C, c. 120 dph精巢切片;D, d. 150 dph精巢切片;E, e. 185 dph 精巢切片;F, f. 210 dph精巢切片;G, g. 360 dph精巢切片;SG. 精原细胞;PSC. 初级精母细胞;SSC. 次级精母细胞;ST. 精细胞;SP. 精子;BC. 血细胞
Fig. 3 The histologic changes of first annual testicular development of Rachycentron canadum
A, a. Section of testis at 60 dph; B, b. section of testis at 90 dph; C, c. section of testis at 120 dph; D, d. section of testis at 150 dph; E, e. section of testis at 185 dph; F, f. section of testis at 210 dph; G, g. section of testis at 360 dph; SG.spermatogonia; PSC. primary spermatocytes; SSC. secondary spermatocytes; ST. spermatids; SP. sperms; BC. blood cells
图 4 军曹鱼卵巢首周年发育的组织学变化
A, a. 60 dph卵巢切片;B, b. 90 dph卵巢切片;C, c. 120 dph卵巢切片;D, d. 150 dph卵巢切片;E, e. 185 dph卵巢切片;F, f. 210 dph卵巢切片;G, g. 360 dph卵巢切片;OG. 卵原细胞;I. 第I时相卵母细胞;II. 第II时相卵母细胞;III. 第III时相卵母细胞;NU. 核仁;OD. 油滴;YN. 卵黄核
Fig. 4 The histologic changes of first annual ovarian development of Rachycentron canadum
A, a. Section of ovary at 60 dph; B, b. section of ovary at 90 dph; C, c. section of ovary at 120 dph; D, d. section of ovary at 150 dph; E, e. section of ovary at 185 dph; F, f. section of ovary at 210 dph; G, g. section of ovary at 360 dph; OG. oogonium; I. oocyte at Stage I; II. oocyte at Stage II; III. oocyte at Stage III; NU. nucleolus; OD. oil droplet; YN. yolk nucleus
-
[1] Castellanos-Galindo G A, Baos R, Zapata L A. Mariculture-induced introduction of cobia Rachycentron canadum (Linnaeus, 1766), a large predatory fish, in the Tropical Eastern Pacific[J]. BioInvasions Records, 2016, 5(1): 55−58. doi: 10.3391/bir.2016.5.1.10 [2] Hamilton S, Severi W, Cavalli R O. Biology and aquaculture of cobia: a review[J]. Boletim do Instituto de Pesca, 2013, 39(4): 461−477. [3] 宋卉, 王树迎. 鱼类原始生殖细胞的研究进展[J]. 动物医学进展, 2004, 25(5): 22−23. doi: 10.3969/j.issn.1007-5038.2004.05.007Song Hui, Wang Shuying. Progress in fish primordial germ cells[J]. Progress in Veterinary Medicine, 2004, 25(5): 22−23. doi: 10.3969/j.issn.1007-5038.2004.05.007 [4] 施瑔芳. 我国鱼类生殖生理学研究概况[J]. 海洋与湖沼, 1992, 23(3): 325−333.Shi Quanfang. An outline of advances on reproductive physiology of fish in China[J]. Oceanologia et Limnologia Sinica, 1992, 23(3): 325−333. [5] Nishimura T, Tanaka M. Gonadal development in fish[J]. Sexual Development, 2014, 8(5): 252−261. doi: 10.1159/000364924 [6] Sajeevan M K, Kurup B M. Age and growth of cobia, Rachycentron canadum (Linnaeus, 1766) occurring in North West Coast of India[J]. Indian Journal of Geo Marine Sciences, 2017, 46(7): 1390−1395. [7] Babatunde T A, Amin S M N, Romano N, et al. Gonad maturation and spawning of cobia, Rachycentron canadum (Linnaeus, 1766) off the Dungun coast, Malaysia[J]. Journal of Applied Ichthyology, 2018, 34(3): 638−645. doi: 10.1111/jai.13650 [8] 许乐乐, 刘楚吾, 刘峰. 雄鱼性腺发育的组织学观察研究进展及生殖上皮(Germinal Epithelium)的作用[J]. 水产科技, 2009(1): 6−13.Xu Lele, Liu Chuwu, Liu Feng. The advances on the research of histological observations to the gonadaldevelopment in male fishes and the effect of germinal epithelium[J]. Fisheries Science & Technology, 2009(1): 6−13. [9] Dhanasekar K, Selvakumar N, Munuswamy N. Ultrastructure of spermatozoa in cobia, Rachycentron canadum (Linnaeus, 1766)[J]. Animal Reproduction Science, 2018, 189: 43−50. doi: 10.1016/j.anireprosci.2017.12.005 [10] Dutney L, Elizur A, Lee P. Analysis of sexually dimorphic growth in captive reared cobia (Rachycentron canadum) and the occurrence of intersex individuals[J]. Aquaculture, 2017, 468: 348−355. doi: 10.1016/j.aquaculture.2016.09.044 [11] 刘筠. 中国养殖鱼类繁殖生理学[M]. 北京: 农业出版社, 1993: 20-42.Liu Yun. Propagation Physiology of Main Cultivated Fish in China[M]. Beijing: Agricultural Publishing Press, 1993: 20-42. [12] 刘少军. 革胡子鲇原始生殖细胞的起源、迁移及性腺分化[J]. 水生生物学报, 1991, 15(1): 1−7.Liu Shaojun. Studies on the orgin and migration of the primordial germ cells and gonad differentiation in Clarias lazera[J]. Acta Hydrobiologica Sinica, 1991, 15(1): 1−7. [13] Zhao Chunyan, Xu Shihong, Liu Yifan, et al. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus)[J]. Fish Physiology and Biochemistry, 2017, 43(1): 265−278. doi: 10.1007/s10695-016-0284-5 [14] Yang Yang, Liu Qinghua, Xiao Yongshuang, et al. Germ cell migration, proliferation and differentiation during gonadal morphogenesis in all-female Japanese flounder (Paralichthys olivaceus)[J]. The Anatomical Record, 2018, 301(4): 727−741. doi: 10.1002/ar.23698 [15] 高书堂, 高令秋, 岳朝霞. 泥鳅原始生殖细胞的发生、迁移和性腺分化[J]. 武汉大学学报(自然科学版), 1998, 44(4): 477−480.Gao Shutang, Gao Lingqiu, Yue Chaoxia. Studies on the origin and migration of the primordial germ cells and gonad differentiation in the loach (Misgurnus anguillicaudatus) embryo[J]. Journal of Wuhan University (Natural Science Edition), 1998, 44(4): 477−480. [16] 游秀容, 蔡明夷, 姜永华, 等. 大黄鱼性腺性别分化的组织学观察[J]. 水产学报, 2012, 36(7): 1057−1064. doi: 10.3724/SP.J.1231.2012.27858You Xiurong, Cai Mingyi, Jiang Yonghua, et al. Histological observation on gonadal sex differentiation in large yellow croaker (Larimichthys crocea)[J]. Journal of Fisheries of China, 2012, 36(7): 1057−1064. doi: 10.3724/SP.J.1231.2012.27858 [17] 代丽. 稀有鮈鲫原始生殖细胞的起源、迁移和分化及卵巢发育和卵子发生研究[D]. 重庆: 西南大学, 2013.Dai Li. Studies on the PGC’s origin migration and differentiation of Gobiocypris rarus and its ovarian development and oogenesis[D]. Chongqing: Southwest University, 2013. [18] Saito D, Morinaga C, Aoki Y, et al. Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai[J]. Developmental Biology, 2007, 310(2): 280−290. doi: 10.1016/j.ydbio.2007.07.039 [19] Lewis Z R, McClellan M C, Postlethwait J H, et al. Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus)[J]. Journal of Morphology, 2008, 269(8): 909−921. doi: 10.1002/jmor.10608 [20] Gao Zexia, Wang Hanping, Rapp D, et al. Gonadal sex differentiation in the bluegill sunfishLepomis macrochirus and its relation to fish size and age[J]. Aquaculture, 2009, 294(1/2): 138−146. [21] Sandra G E, Norma M M. Sexual determination and differentiation in teleost fish[J]. Reviews in Fish Biology and Fisheries, 2009, 20(1): 101−121. [22] Brown-Peterson N J, Overstreet R M, Lotz J M, et al. Reproductive biology of cobia, Rachycentron canadum, from coastal waters of the southern United States[J]. Fishery Bulletin, 2001, 99(1): 15−28. [23] Lotz J M, Overstreet R M, Franks J S. Gonadal maturation in the cobia, Rachycentron canadum, from the northcentral gulf of mexico[J]. Gulf Research Reports, 1996, 9(3): 147−159. [24] van der Velde T D, Griffiths S P, Fry G C. Reproductive biology of the commercially and recreationally important cobia Rachycentron canadum in northeastern Australia[J]. Fisheries Science, 2010, 76(1): 33−43. doi: 10.1007/s12562-009-0177-y [25] 蓝军南, 区又君, 温久福, 等. 四指马鲅精巢发育及精子发生的组织学和超微结构[J]. 中国水产科学, 2020, 27(6): 637−648.Lan Junnan, Ou Youjun, Wen Jiufu, et al. Histology and ultrastructure of developing testes and spermatogenesis in the fourfinger threadfin, Eleutheronema tetradactylum (Show, 1804)[J]. Journal of Fishery Sciences of China, 2020, 27(6): 637−648. [26] 黄小林, 杨育凯, 李涛, 等. 池塘养殖黄斑篮子鱼初次性成熟性腺发育研究[J]. 南方水产科学, 2020, 16(5): 99−107. doi: 10.12131/20200051Huang Xiaolin, Yang Yukai, Li Tao, et al. Gonadal development of first sexual maturation of Siganus oramin cultured in pond[J]. South China Fisheries Science, 2020, 16(5): 99−107. doi: 10.12131/20200051 [27] 崔丹, 刘志伟, 刘南希, 等. 金钱鱼性腺发育及其组织结构观察[J]. 水产学报, 2013, 37(5): 696−704. doi: 10.3724/SP.J.1231.2013.38442Cui Dan, Liu Zhiwei, Liu Nanxi, et al. Histological study on the gonadal development of Scatophagus argus[J]. Journal of Fisheries of China, 2013, 37(5): 696−704. doi: 10.3724/SP.J.1231.2013.38442 [28] 赵一杰, 张美昭, 温海深. 松江鲈鱼性腺发育组织学观察[J]. 海洋湖沼通报, 2013(1): 16−22.Zhao Yijie, Zhang Meizhao, Wen Haishen. Histological observation of gonad development in Trachidermus fasciatus[J]. Transactions of Oceanology and Limnology, 2013(1): 16−22. [29] Schulz R W, de Franca L R, Lareyre J J, et al. Spermatogenesis in fish[J]. General and Comparative Endocrinology, 2010, 165(3): 390−411. doi: 10.1016/j.ygcen.2009.02.013 [30] 刘晨斌, 徐革锋, 黄天晴, 等. 鱼类性腺发育研究进展[J]. 水产学杂志, 2019, 32(1): 46−54. doi: 10.3969/j.issn.1005-3832.2019.01.009Liu Chenbin, Xu Gefeng, Huang Tianqing, et al. A review of research progress on gonadal development in fish[J]. Chinese Journal of Fisheries, 2019, 32(1): 46−54. doi: 10.3969/j.issn.1005-3832.2019.01.009 [31] 刘皓, 张玉红, 罗杰, 等. 红鳍笛鲷(Lutjanus erythopterus)卵巢发育的组织学研究[J]. 海洋与湖沼, 2016, 47(1): 269−275.Liu Hao, Zhang Yuhong, Luo Jie, et al. Histology of ovarian development of crimson snapper Lutjanus erythopterus[J]. Oceanologia et Limnologia Sinica, 2016, 47(1): 269−275. [32] 柳学周, 徐永江, 刘乃真, 等. 半滑舌鳎卵巢发育的组织学和形态数量特征研究[J]. 渔业科学进展, 2009, 30(6): 25−35. doi: 10.3969/j.issn.1000-7075.2009.06.004Liu Xuezhou, Xu Yongjiang, Liu Naizhen, et al. Study on histological and morphometric characters of gonad development of Cynoglossus semilaevis Günther [J]. Progress in Fishery Sciences, 2009, 30(6): 25−35. doi: 10.3969/j.issn.1000-7075.2009.06.004 [33] 李培伦, 刘伟, 王继隆. 乌苏里白鲑洄游群体性腺发育组织学观察[J]. 生物学杂志, 2015, 32(1): 34−38. doi: 10.3969/j.issn.2095-1736.2015.01.034Li Peilun, Liu Wei, Wang Jilong. Observation on the gonadal development of the Siberian gudgeon migratory populations[J]. Journal of Biology, 2015, 32(1): 34−38. doi: 10.3969/j.issn.2095-1736.2015.01.034 [34] 孙鹏, 尹飞, 施兆鸿, 等. 养殖银鲳卵巢发育的组织学观察[J]. 中国水产科学, 2013, 20(2): 293−298. doi: 10.3724/SP.J.1118.2013.00293Sun Peng, Yin Fei, Shi Zhaohong, et al. Histological analysis of ovary development in the cultured silver pom-fret, Pampus argenteus[J]. Journal of Fishery Sciences of China, 2013, 20(2): 293−298. doi: 10.3724/SP.J.1118.2013.00293 [35] 施兆鸿, 罗海忠, 高露姣, 等. 灰鲳卵巢发育的组织学研究[J]. 海洋水产研究, 2006, 27(4): 1−5.Shi Zhaohong, Luo Haizhong, Gao Lujiao, et al. Study on histology of ovary development of Pampus cinereus[J]. Marine Fisheries Research, 2006, 27(4): 1−5. [36] 史丹, 温海深, 杨艳平. 许氏平鲉卵巢发育的周年变化研究[J]. 中国海洋大学学报, 2011, 41(9): 25−30.Shi Dan, Wen Haishen, Yang Yanping. The annual change of ovarian development in female Sebastes schlegeli[J]. Periodical of Ocean University of China, 2011, 41(9): 25−30. [37] 朱亮华, 孙敏, 张鼎元, 等. 黑鱾卵巢发育的组织学研究[J]. 应用海洋学学报, 2018, 37(2): 255−262. doi: 10.3969/J.ISSN.2095-4972.2018.02.013Zhu Lianghua, Sun Min, Zhang Dingyuan, et al. Histological study on the ovary development of Girella leonina[J]. Journal of Applied Oceanography, 2018, 37(2): 255−262. doi: 10.3969/J.ISSN.2095-4972.2018.02.013 [38] 洪磊, 李兆新, 陈超, 等. 美洲鲥鱼卵巢发育规律和性类固醇激素变化研究[J]. 中国工程科学, 2014, 16(9): 86−92. doi: 10.3969/j.issn.1009-1742.2014.09.013Hong Lei, Li Zhaoxin, Chen Chao, et al. The study of ovary development and steroid hormone changes in Alosa sapidissima[J]. Engineering Science, 2014, 16(9): 86−92. doi: 10.3969/j.issn.1009-1742.2014.09.013 -




 下载:
下载:



