Effects of Spartina alterniflora invasion on mercury speciation in vegetated sediments of the wetland in Changjiang River Estuary, China
-
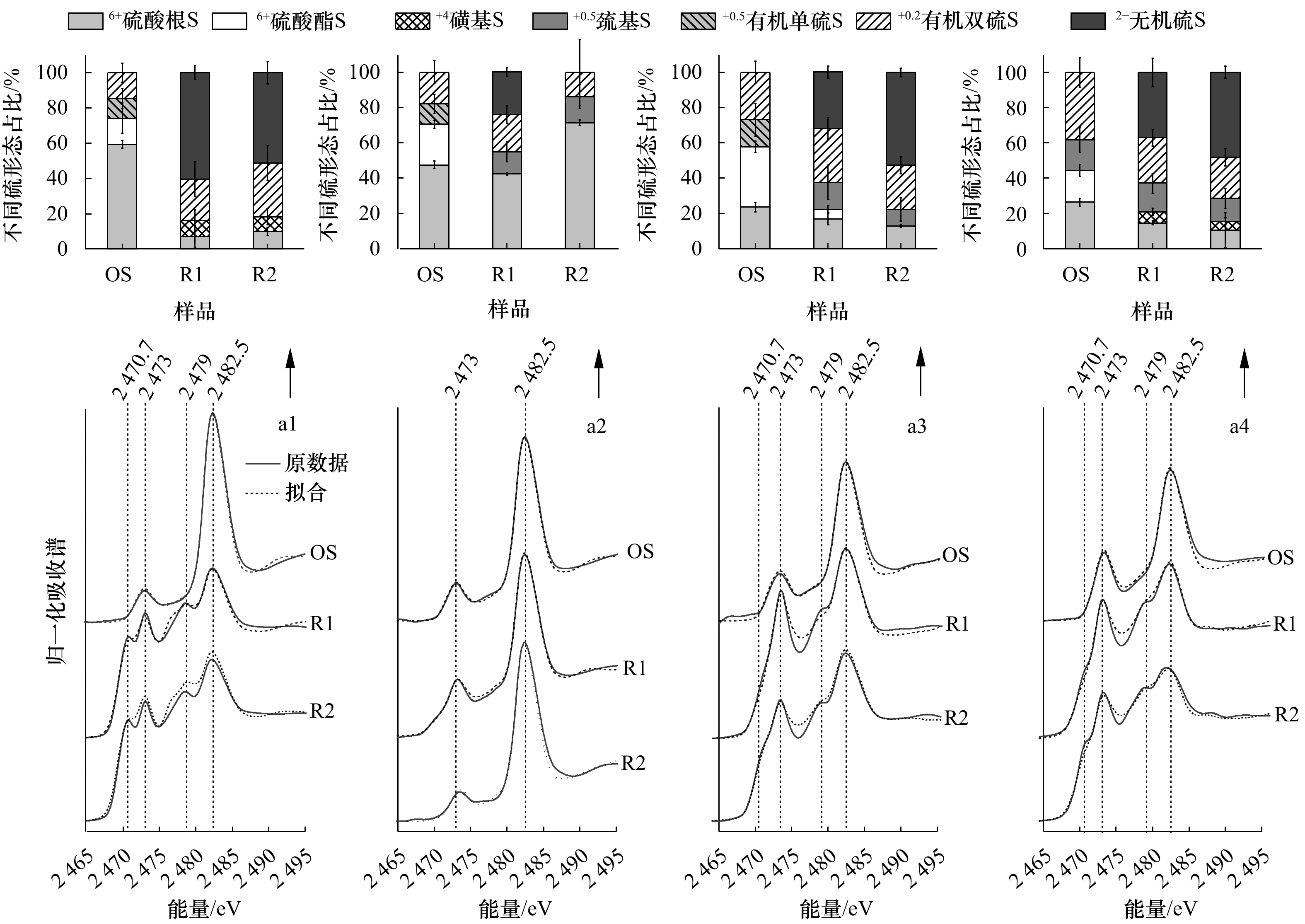
摘要: 通过分析长江河口湿地典型植物根际沉积物柱样(0~40 cm)中总汞(THg)、甲基汞(MeHg)及其与粒度、总有机碳(TOC)、还原态硫等环境因子之间的关系,探讨了互花米草(Spartina alterniflora)入侵对沉积物中汞形态特征的影响及主控因子。结果表明:(1)不同植物(互花米草、芦苇(Phragmites communis)、海三棱藨草(Scirpus mariqueter)和水葱(Scirpus tabernaemontani))根际沉积物中THg均值为49.9~100.9 μg/kg,其与粒径小于16 µm颗粒物组分体积百分比及TOC含量之间存在显著正相关关系(r2=0.85,p<0.01;r2=0.58,p<0.01),这意味着沉积物中矿物−有机物复合体细颗粒物的空间分异决定着总汞的空间分异。互花米草入侵促进了细颗粒的沉积,进而间接促进了沉积物中总汞含量的增加。(2)不同植物根际沉积物中MeHg均值为0.3~1.4 μg/kg,MeHg/THg均值为0.4%~1.4%,互花米草、芦苇及海三棱藨草根际沉积物中MeHg含量及MeHg/THg值随深度增加不断减小,但无显著差异,表明了互花米草入侵对沉积物中汞甲基化过程的影响可能有限。Pearson相关分析表明,MeHg/THg与THg、TOC、酸挥发性硫之间不存在显著的正相关关系。硫的K边同步辐射结果进一步表明了硫形态(如有机硫和S2−)变化与MeHg变化关系不大。MeHg/THg值呈表层(0~8 cm)高,底层低的分布规律,表明了表层沉积物中汞的甲基化潜势较大,这可能与表层新鲜有机质(如藻类和植物凋落物)的不断供给及其降解过程密切相关,还需深入研究。Abstract: To investigate the changes in mercury (Hg) speciation in vegetated sediments of the wetlands in Changjiang River Estuary, China, following the invasion of Spartina alterniflora, we determined total mercury (THg), methylmercury (MeHg), toatal organic carbon (TOC), reduced sulfur (S) and grain size in core sediments (0−40 cm) vegetated with different plants in wetlands. The results showed that: (1) the mean concentrations of THg were 49.9−100.9 μg/kg in sediments vegetated by S. alterniflora,
Phragmites communis, Scirpus mariqueter and Scirpus tabernaemontani had a significant positive correlation with the fraction of fine particles (<16 µm) and TOC content (r2=0.85, p<0.01; r2 =0.58, p<0.01), indicating that the distribution of Hg levels in sediments could be dominated by the spatial differentiation of the mineral-organic complexes in fine particles. The invasion of S. alterniflora promoted the deposition of fine particles, and thus could facilitate Hg storage indirectly in wetland sediments. (2) The mean concentrations MeHg and average values of MeHg/THg (%) in vegetated sediments were 0.3−1.4 μg/kg and 0.4%−1.4%, respectively. The profiles of MeHg and MeHg/THg exhibited decrease with increasing depth across all sites. There was no significant difference in the content of MeHg and the values of MeHg/THg in vegetated sediments dominated by S. alterniflora, P. australis and S. mariqueter, indicating that the impact of S. alterniflora invasion on Hg methylation may be limited. In addition, there was no significant positive correlation between MeHg/THg and THg, TOC and acid volatile sulfur (AVS). S-K edge XANES further revealed that the reduced sulfur (S) (e. g., thoil and sulfide) changed greatly in depth profiles. These results suggested that the changes in reduced sulfur could have limited impacts on MeHg production. The values of MeHg/THg was higher in surface horizon (0~8 cm) than in deeper horizon, indicating that the higher rates of Hg methylation in surface sediments and the degradation of fresh organic matter (e. g., algae and plant litter) could be the key biogeochemical process on controlling MeHg production in surface sediments of wetland in Changjiang River Estuary. -
Key words:
- sediment /
- mercury /
- acid volatile sulfur /
- spartina alterniflora /
- Changjiang River Estuary /
- wetland
-
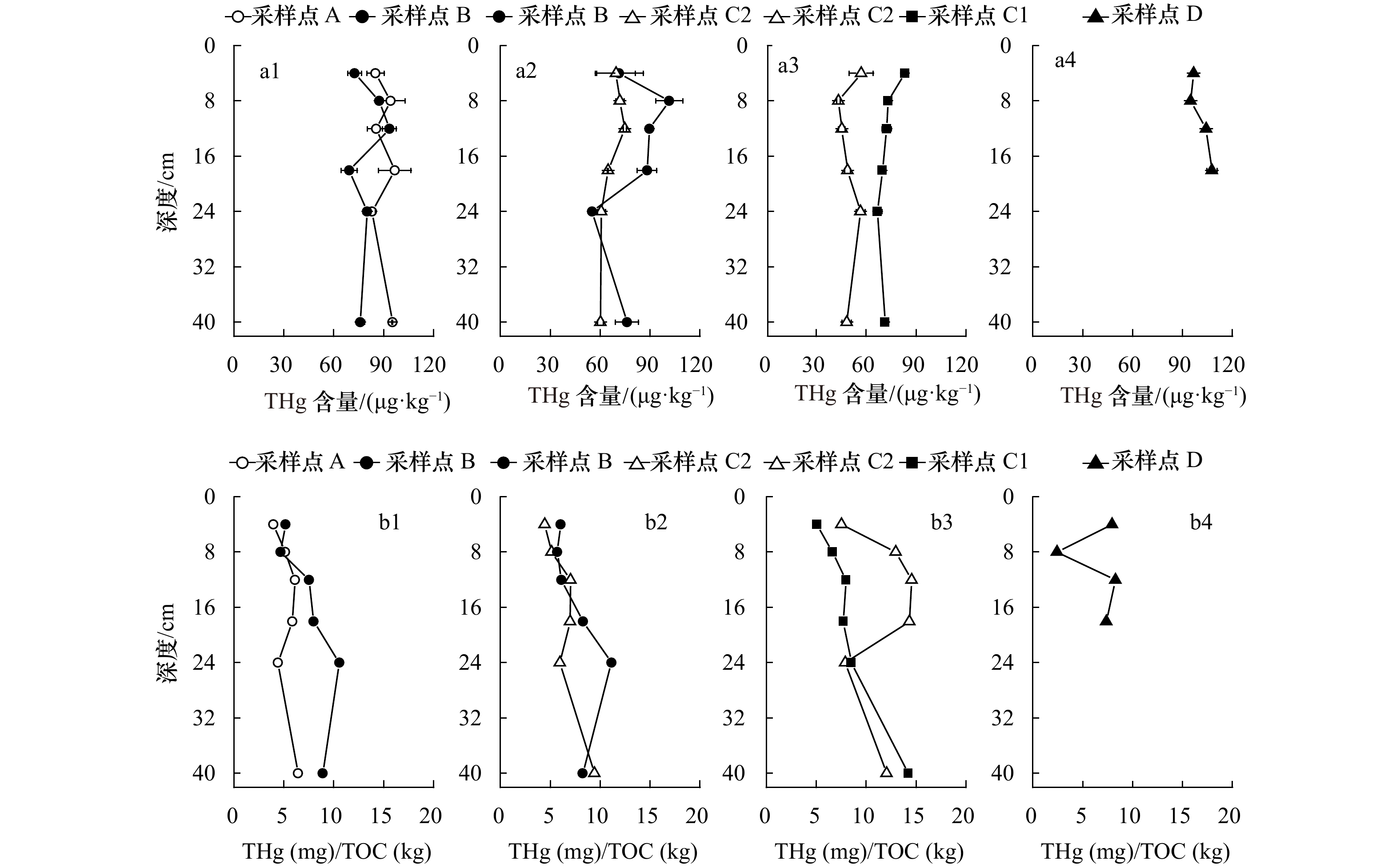
图 2 长江河口湿地不同植物根际沉积物中THg含量与THg/TOC值特征
a1, b1. 互花米草;a2, b2. 芦苇;a3, b3. 海三棱藨草;a4, b4. 水葱
Fig. 2 Concentrations of total Hg and THg/TOC in vegetated sediments in different wetlands, Changjiang River Estuary
a1, b1. Spartina alterniflora; a2, b2. Phragmites australis; a3, b3. Scirpus mariqueter; a4, b4. Scirpus tabernaemontani
图 3 长江河口湿地不同植物根际柱状沉积物中MeHg含量与MeHg/THg特征
a1, b1. 互花米草;a2, b2. 芦苇;a3, b3. 海三棱藨草;a4, b4. 水葱
Fig. 3 MeHg concentrations and MeHg/THg in vegetated sediments in different wetlands, Changjiang River Estuary
a1, b1. Spartina alterniflora; a2, b2. Phragmites australis; a3, b3. Scirpus mariqueter; a4, b4. Scirpus tabernaemontani
图 6 不同植物根际沉积物TOC与THg(a)及THg/TOC(b)关系
拟合不包含三角和圆圈数据;三角是D点4~8 cm深度样品;圆圈是文献[26]的研究结果
Fig. 6 Relationships between TOC and THg (a) and THg/TOC (b) in vegetated sediments
The fitting does not contain the date of triangular and circle; the triangular indicates the sample from the depths of 4~8 cm in site D; the open circle indicates the data from reference[26]
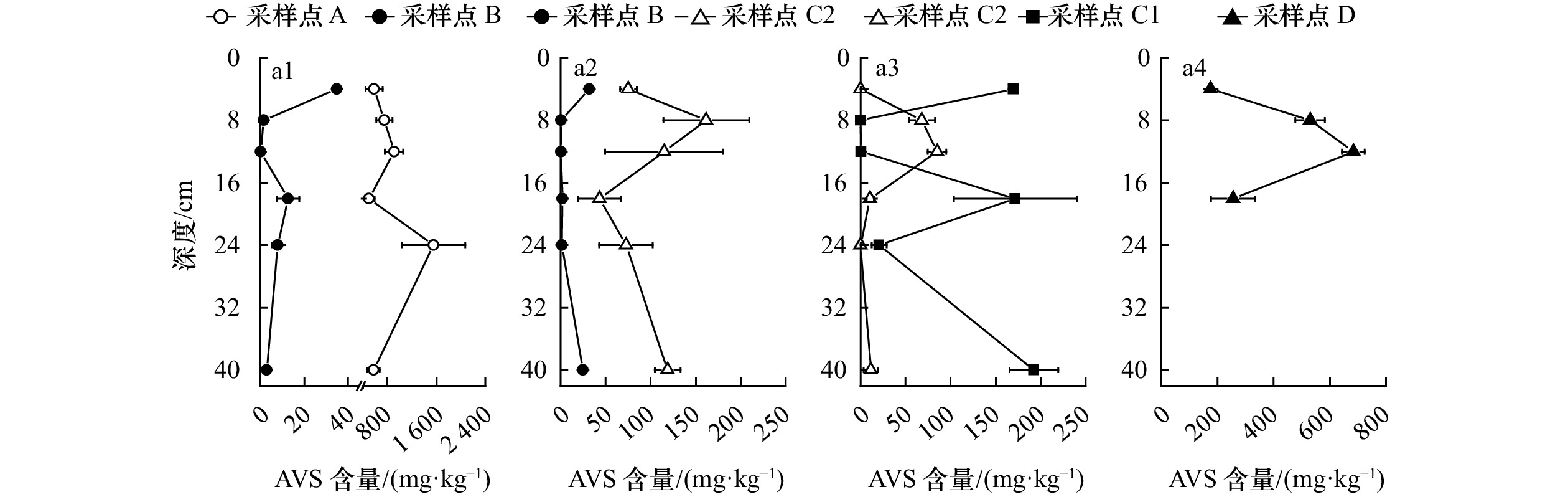
表 1 长江河口湿地不同植物根际柱状沉积物理化特征
Tab. 1 Physical and chemical parameters of vegetated sediments in wetlands, Changjiang River Estuary
植被类型 E EN EN EN E E E 粒径小于
16 µm体积
百分比/%TOC/% THg含量
/(μg·kg−1)THg(mg)/
TOC(kg)MeHg含量
/(μg·kg−1)AVS含量
/(mg·kg−1)(MeHg/Hg)
/%互花米草
(Spartina alterniflora)A点,高潮滩(n=6) 81.0±2.9a 1.7±0.3a 90.0±6.0a 5.3±1.0b 0.5±0.3 811.4±392.4a 0.6±0.4 B点,中潮滩(n=6) 66.4±3.9b 1.2±0.4ab 79.8±9.1ab 7.5±2.2ab 0.5±0.2 10.1±13.0c 0.6±0.3 芦苇
(Phragmites australis)B点,中潮滩(n=6) 66.1±11.7b 1.3±0.4ab 80.4±16.3ab 6.5±1.8b 0.4±0.3 10.1±14.3c 0.5±0.3 C2点,中潮滩(n=6) 54.7±6.5bc 0.9±0.3bc 67.0±6.0b 7.6±2.1ab 0.3±0.1 97.8±42.3b 0.4±0.1 海三棱藨草
(Scirpus mariqueter)C1点,中潮滩(n=6) 58.5±5.7bc 1.0±0.4bc 72.8±5.6b 8.3±3.1ab 0.4±0.4 92.5±94.3bc 0.6±0.5 C2点,中潮滩前缘(n=6) 34.6±5.3c 0.5±0.2c 49.9±5.7c 11.6±3.1a 0.4±0.3 29.2±37.3c 0.9±0.6 水葱(Scirpus
tabernaemontani)D点,中潮滩前缘(n=4) 80.5±1.7 2.0±1.3 100.9±6.1 6.5±2.7 1.4±1.4 411.6±236.4 1.4±1.5 注:表中E,EN分别表示数据方差齐(equal variances assumed)和方差不齐(equal variances not assumed),不同字母a,b,c表示差异显著(p<0.05),无字母标注和含有相同字母表示无显著差异(p>0.05)。由于水葱主要生长在盐度较低的横沙岛湿地,故主要对崇明东滩湿地不同植物根际沉积物中各理化指标进行对比分析。 -
[1] Hsu-Kim H, Kucharzyk K H, Zhang Tong, et al. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review[J]. Environmental Science & Technology, 2013, 47(6): 2441−2456. [2] 冯新斌, 史建波, 李平, 等. 我国汞污染研究与履约进展[J]. 中国科学院院刊, 2020, 35(11): 1344−1350.Feng Xinbin, Shi Jianbo, Li Ping, et al. Progress of mercury pollution research and implementation of minamata convention in China[J]. Bulletin of Chinese Academy of Sciences, 2020, 35(11): 1344−1350. [3] 丁振华, 刘金铃, 李柳强, 等. 中国主要红树林湿地沉积物中汞的分布特征[J]. 环境科学, 2009, 30(8): 2210−2215. doi: 10.3321/j.issn:0250-3301.2009.08.005Ding Zhenhua, Liu Jinling, Li Liuqiang, et al. Distribution of mercury in surficial sediments from main mangrove wetlands of China[J]. Environmental Science, 2009, 30(8): 2210−2215. doi: 10.3321/j.issn:0250-3301.2009.08.005 [4] 王起超, 刘汝海, 吕宪国, 等. 湿地汞环境过程研究进展[J]. 地球科学进展, 2002, 17(6): 881−885. doi: 10.3321/j.issn:1001-8166.2002.06.013Wang Qichao, Liu Ruhai, Lü Xianguo, et al. Progress of study on the mercury process in the wetland environment[J]. Advance in Earth Sciences, 2002, 17(6): 881−885. doi: 10.3321/j.issn:1001-8166.2002.06.013 [5] Krabbenhoft D P, Sunderland E M. Global change and mercury[J]. Science, 2013, 341(6153): 1457−1458. doi: 10.1126/science.1242838 [6] United Nations Environment Programme (UNEP). Global mercury assessment 2018[EB/OL]. (2019-03-04)[ 2020-03-04]. https://www.unep.org/resources/publication/global-mercury-assessment−2018. [7] Jonsson S, Skyllberg U, Nilsson M B, et al. Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota[J]. Nature Communications, 2014, 5: 4624. doi: 10.1038/ncomms5624 [8] 宋连环, 郑祥民, 周立旻, 等. 崇明东滩湿地沉积物中汞累积特征及其影响因素研究[J]. 环境科学研究, 2009, 22(12): 1426−1432.Song Lianhuan, Zheng Xiangmin, Zhou Limin, et al. Cumulation characteristics and influencing factors of mercury in sediments from Chongming wetland[J]. Research of Environmental Sciences, 2009, 22(12): 1426−1432. [9] Deng Huanguang, Wang Dongqi, Chen Zhenlou, et al. A comprehensive investigation and assessment of mercury in intertidal sediment in continental coast of Shanghai[J]. Environmental Science and Pollution Research, 2013, 20(9): 6297−6305. doi: 10.1007/s11356-013-1665-2 [10] Li Bo, Liao Chengzhang, Zhang Xiaodong, et al. Spartina alterniflora invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects[J]. Ecological Engineering, 2009, 35(4): 511−520. doi: 10.1016/j.ecoleng.2008.05.013 [11] Nie Ming, Wang Meng, Li Bo. Effects of salt marsh invasion by Spartina alterniflora on sulfate-reducing bacteria in the Yangtze River estuary, China[J]. Ecological Engineering, 2009, 35(12): 1804−1808. doi: 10.1016/j.ecoleng.2009.08.002 [12] Zeleke J, Sheng Qiang, Wang Jiangong, et al. Effects of Spartina alterniflora invasion on the communities of methanogens and sulfate-reducing bacteria in estuarine marsh sediments[J]. Frontiers in Microbiology, 2013, 4: 243. [13] 汤臣栋. 上海崇明东滩互花米草生态控制与鸟类栖息地优化工程[J]. 湿地科学与管理, 2016, 12(3): 4−8. doi: 10.3969/j.issn.1673-3290.2016.03.01Tang Chendong. Ecological control of Spartina alterniflora and improvement of birds habitats in Chongming Dongtan wetland, Shanghai[J]. Wetland Science & Management, 2016, 12(3): 4−8. doi: 10.3969/j.issn.1673-3290.2016.03.01 [14] Liang Lian, Horvat M, Feng Xinbin, et al. Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples[J]. Applied Organometallic Chemistry, 2004, 18(6): 264−270. doi: 10.1002/aoc.617 [15] DeWild J F, Olund S D, Olson M L, et al. Methods for the Preparation and Analysis of Solids and Suspended Solids for Methylmercury, Chapter 7 of Book 5, Laboratory Analysis Section A, Water Analysis[M]. Reston, Virginia: U. S. Geological Survey, 2004: 1−12. [16] Burton E D, Sullivan L A, Bush R T, et al. A simple and inexpensive chromium-reducible sulfur method for acid-sulfate soils[J]. Applied Geochemistry, 2008, 23(9): 2759−2766. doi: 10.1016/j.apgeochem.2008.07.007 [17] Sunderland E M, Gobas F A P C, Heyes A, et al. Speciation and bioavailability of mercury in well-mixed estuarine sediments[J]. Marine Chemistry, 2004, 90(1/4): 91−105. [18] Prietzel J, Tyufekchieva N, Eusterhues K, et al. Anoxic versus oxic sample pretreatment: effects on the speciation of sulfur and iron in well-aerated and wetland soils as assessed by x-ray absorption near-edge spectroscopy (XANES)[J]. Geoderma, 2009, 153(3/4): 318−330. [19] Mason R P, Choi A L, Fitzgerald W F, et al. Mercury biogeochemical cycling in the ocean and policy implications[J]. Environmental Research, 2012, 119: 101−117. doi: 10.1016/j.envres.2012.03.013 [20] Jang J, Kim H, Han S. Influence of microorganism content in suspended particles on the particle-water partitioning of mercury in semi-enclosed coastal waters[J]. Science of the Total Environment, 2014, 470−471: 1558−1564. doi: 10.1016/j.scitotenv.2013.08.097 [21] Zhang Lijie, Wu Shan, Zhao Linduo, et al. Mercury sorption and desorption on organo-mineral particulates as a source for microbial methylation[J]. Environmental Science & Technology, 2019, 53(5): 2426−2433. [22] Yang S L, Li H, Ysebaert T, et al. Spatial and temporal variations in sediment grain size in tidal wetlands, Yangtze Delta: on the role of physical and biotic controls[J]. Estuarine, Coastal and Shelf Science, 2008, 77(4): 657−671. doi: 10.1016/j.ecss.2007.10.024 [23] Obrist D, Johnson D W, Lindberg S E, et al. Mercury distribution across 14 U. S. forests. Part I: spatial patterns of concentrations in biomass, litter, and soils[J]. Environmental Science & Technology, 2011, 45(9): 3974−3981. [24] Chakraborty P, Sarkar A, Vudamala K, et al. Organic matter—a key factor in controlling mercury distribution in estuarine sediment[J]. Marine Chemistry, 2015, 173: 302−309. doi: 10.1016/j.marchem.2014.10.005 [25] Skyllberg U, Bloom P R, Qian Jian, et al. Complexation of mercury(II) in soil organic matter: EXAFS evidence for linear two-coordination with reduced sulfur groups[J]. Environmental Science & Technology, 2006, 40(13): 4174−4180. [26] Chen Hongzhe, Wang Jigang, Chen Jinmin, et al. Assessment of heavy metal contamination in the surface sediments: a reexamination into the offshore environment in China[J]. Marine Pollution Bulletin, 2016, 113(1/2): 132−140. [27] 顿佳耀, 王初, 姚东京, 等. 崇明东滩盐沼表层沉积物有机碳空间分布特征及其来源示踪研究[J]. 长江流域资源与环境, 2019, 28(1): 157−165.Dun Jiayao, Wang Chu, Yao Dongjing, et al. Spatial distribution characteristics and source tracing of organic carbon in surface sediments of salt marsh in Dongtan of Chongming[J]. Resources and Environment in the Yangtze Basin, 2019, 28(1): 157−165. [28] Grasby S E, Them T R II, Chen Zhuoheng, et al. Mercury as a proxy for volcanic emissions in the geologic record[J]. Earth-Science Reviews, 2019, 196: 102880. doi: 10.1016/j.earscirev.2019.102880 [29] Xue Wen, Kwon S Y, Grasby S E, et al. Anthropogenic influences on mercury in Chinese soil and sediment revealed by relationships with total organic carbon[J]. Environmental Pollution, 2019, 255: 113186. doi: 10.1016/j.envpol.2019.113186 [30] Schartup A T, Mason R P, Balcom P H, et al. Methylmercury production in estuarine sediments: role of organic matter[J]. Environmental Science & Technology, 2013, 47(2): 695−700. [31] Noh S, Choi M, Kim E, et al. Influence of salinity intrusion on the speciation and partitioning of mercury in the Mekong River Delta[J]. Geochimica et Cosmochimica Acta, 2013, 106: 379−390. doi: 10.1016/j.gca.2012.12.018 [32] Rickard D, Morse J W. Acid volatile sulfide (AVS)[J]. Marine Chemistry, 2005, 97(3/4): 141−197. [33] Ouddane B, Mikac N, Cundy A B, et al. A comparative study of mercury distribution and methylation in mudflats from two macrotidal estuaries: the Seine (France) and the Medway (United Kingdom)[J]. Applied Geochemistry, 2008, 23(4): 618−631. doi: 10.1016/j.apgeochem.2007.11.001 [34] Jonsson S, Skyllberg U, Nilsson M B, et al. Mercury methylation rates for geochemically relevant HgII species in sediments[J]. Environmental Science & Technology, 2012, 46(21): 11653−11659. [35] Marvin-DiPasquale M, Agee J, McGowan C, et al. Methyl-mercury degradation pathways: a comparison among three mercury-impacted ecosystems[J]. Environmental Science & Technology, 2000, 34(23): 4908−4916. [36] Kim M, Han S, Gieskes J, et al. Importance of organic matter lability for monomethylmercury production in sulfate-rich marine sediments[J]. Science of the Total Environment, 2011, 409(4): 778−784. doi: 10.1016/j.scitotenv.2010.10.050 [37] Bravo A G, Bouchet S, Tolu J, et al. Molecular composition of organic matter controls methylmercury formation in boreal lakes[J]. Nature Communications, 2017, 8: 14255. doi: 10.1038/ncomms14255 -





 下载:
下载: