Effects of ocean acidification and warming on the larvae settlement and post-settlement survival of two reef-building corals
-
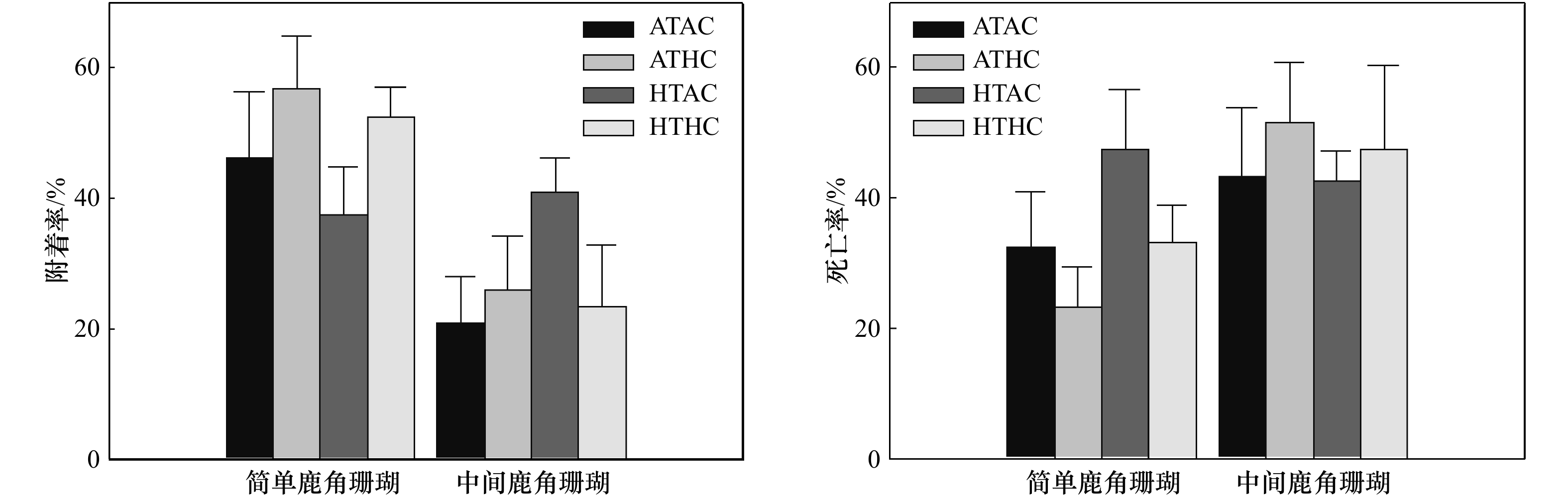
摘要: 大气CO2浓度持续升高导致海洋酸化和暖化影响着造礁石珊瑚和珊瑚礁生态系统。为探明造礁石珊瑚早期生活史对海洋酸化和暖化的生理学响应,本文研究了温度(约28°C, 约30°C)和pCO2(约570 µatm, 约1 300 μatm)以及两者协同作用对简单鹿角珊瑚(Acropora austera)和中间鹿角珊瑚(A. intermedia)早期生活史的影响。实验结果表明,升温(+约2.5°C)和酸化(约1 300 μatm)对两种鹿角珊瑚幼虫的附着率和死亡率均无显著影响。酸化显著降低了简单鹿角珊瑚幼体存活率(25.87%),但并不显著影响中间鹿角珊瑚幼体的存活率;升温对两种鹿角珊瑚幼体存活率无显著影响。升温(+约2.5°C)、酸化(约1 300 μatm)对简单、中间鹿角珊瑚幼虫的存活和附着过程的影响较小,但是酸化对简单鹿角珊瑚幼体存活的影响高于暖化。本文结果表明,珊瑚补充过程对海洋酸化和暖化的响应可能具有种类特异性,气候变化将逐渐改变造礁石珊瑚的群落结构。Abstract: Ocean acidification and warming are occurring globally through increasing CO2 absorption into the oceans, and impose two serious and imminent threats to the persistence of scleractinian corals and the reef ecosystem they construct. To evaluate the effects of ocean acidification and warming on the early life stages of the reef-building corals Acropora austera and A. intermedia, their larvae were incubated at a full cross design of two temperatures (about 28℃, about 30℃) and pCO2 (about 570 μatm, about 1 300 μatm) for 8 d. There were no significant differences in rates of settlement. Larval mortality rates of two reef-building corals were unaffected in any treatments. High pCO2 significantly reduced post-settlement survival of A. austera but not A. intermedia, with a 25.87% reduction in post-settlement survival in high pCO2 compared to control. Our results show that larvae settlement and mortality rates of the reef-building corals A. austera and A. intermedia were unaffected by ocean acidification and warming, and high pCO2 are more detrimental to mortality rates of juvenile A. austera than high temperature. Different species of juvenile corals exhibit species-specific response to ocean acidification and warming, with important implications for coral recruitment and even species structure composition of reef-building corals in the future ocean.
-
Key words:
- ocean acidification and warming /
- coral /
- larvae settlement rates /
- mortality /
- post-settlement survival
-
表 1 实验期间各个处理海水理化因子的平均水平
Tab. 1 Experimental seawater conditions for each treatment
处理 T/℃ S pH pCO2 /µatm TA/µmol·kg−1 ΩArag ATAC 27.95±0.92 32.98±0.70 8.05±0.07 571.90±14.53 2 224.85±19.43 2.37±0.03 ATHC 27.80±0.21 32.90±0.26 7.72±0.13 1 338.43±43.87 2 212.67±13.14 1.15±0.04 HTAC 30.35±0.80 33.23±0.61 8.05±0.10 576.77±16.76 2 221.67±13.43 2.52±0.05 HTHC 30.05±0.76 33.18±0.31 7.75±0.15 1 290.85±52.03 2 245.83±8.35 1.33±0.04 注:数据表示为均值±标准差。海水理化参数为温度(T)、盐度(S)、pH、二氧化碳分压(pCO2)、总碱度(Total Alkalinity, TA)和文石饱和度(ΩArag)。 表 2 双因素方差分析结果:温度、pCO2对两种珊瑚幼虫附着率、死亡率及附着后存活的影响
Tab. 2 Results of two-way ANOVAs testing the effects of elevated temperature, pCO2 on the percentages of settlement, mortality, post-settlement survival of two coral species
变化因素 离均差平方和 自由度 均方 F统计量 p 简单鹿角珊瑚幼虫附着率 温度 0.034 1 0.034 0.715 0.405 pH 0.131 1 0.131 2.727 0.110 温度和pH 0.004 1 0.004 0.079 0.780 误差 1.348 28 0.048 中间鹿角珊瑚幼虫附着率 温度 0.046 1 0.046 1.277 0.272 pH 0.023 1 0.023 0.651 0.429 温度和pH 0.076 1 0.076 2.111 0.162 误差 0.720 20 0.036 简单鹿角珊瑚幼虫死亡率 温度 0.125 1 0.125 2.757 0.108 pH 0.113 1 0.113 2.488 0.126 温度和pH 0.005 1 0.005 0.110 0.742 误差 1.269 28 0.045 中间鹿角珊瑚幼虫死亡率 温度 0.004 1 0.004 0.066 0.800 pH 0.027 1 0.027 0.468 0.502 温度和pH 0.002 1 0.002 0.029 0.866 误差 1.139 20 0.057 简单鹿角珊瑚幼体存活率 温度 6.1×10−5 1 6.1×10−5 0.001 0.979 pH 0.470 1 0.470 5.424 0.028 温度和pH 0.001 1 0.001 0.013 0.912 误差 2.342 27 0.087 中间鹿角珊瑚幼体存活率 温度 0.046 1 0.046 0.411 0.530 pH 0.067 1 0.067 0.592 0.452 温度和pH 0.219 1 0.219 1.934 0.182 误差 1.922 17 0.113 -
[1] Orr J C. Recent and future changes in ocean carbonate chemistry[M]//Gattuso J P, Hansson L. Ocean Acidification. Oxford: Oxford University Press, 2011: 41−66. [2] Sabine C L, Feely R A, Gruber N, et al. The oceanic sink for anthropogenic CO2[J]. Science, 2004, 305(5682): 367−371. doi: 10.1126/science.1097403 [3] Collins M, Knutti R, Arblaser J, et al. Long-term climate change: projections, commitments and irreversibility[M]//Stocker T F, Qin D, Plattner G K, et al. Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 2013. [4] Friedlingstein P, Andrew R M, Rogelj J, et al. Persistent growth of CO2 emissions and implications for reaching climate targets[J]. Nature Geoscience, 2014, 7(10): 709−715. doi: 10.1038/ngeo2248 [5] IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group Ⅱ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change[M]. Cambridge: Cambridge University Press, 2014. [6] Huang Hui, Yuan Xiangcheng, Cai Weijun, et al. Positive and negative responses of coral calcification to elevated pCO2: case studies of two coral species and the implications of their responses[J]. Marine Ecology Progress Series, 2014, 502: 145−156. doi: 10.3354/meps10720 [7] Watson S A, Southgate P C, Miller G M, et al. Ocean acidification and warming reduce juvenile survival of the fluted giant clam, Tridacna squamosa[J]. Molluscan Research, 2012, 32(3): 177−180. [8] Timmins-Schiffman E, O'Donnell M J, Friedman C S, et al. Elevated pCO2 causes developmental delay in early larval Pacific oysters, Crassostrea gigas[J]. Marine Biology, 2013, 160(8): 1973−1982. doi: 10.1007/s00227-012-2055-x [9] Hughes T P, Kerry J T, Álvarez-Noriega M, et al. Global warming and recurrent mass bleaching of corals[J]. Nature, 2017, 543(7645): 373−377. doi: 10.1038/nature21707 [10] Zheng Xinqing, Kuo Fuwen, Pan Ke, et al. Different calcification responses of two hermatypic corals to CO2-driven ocean acidification[J]. Environmental Science and Pollution Research, 2019, 26(7): 30596−30602 . doi: 10.1007/s11356-018-1376-9 [11] 郑新庆, 郭富雯, 刘昕明, 等. 海洋酸化没有显著影响成体鹿角杯形珊瑚的钙化作用和光合能力[J]. 海洋学报, 2015, 37(10): 59−68.Zheng Xinqing, Kuo Fuwen, Liu Xinming, et al. Ocean acidification does not significantly affect the calcification and photosynthesis capacity of hermatypic coral Pocillopora damicornis[J]. Haiyang Xuebao, 2015, 37(10): 59−68. [12] Cossins A R, Bowler K. Temperature Biology of Animals[M]. Dordrecht, Netherlands: Springer, 1987. [13] Ritson-Williams R, Arnold S N, Fogarty N D, et al. New perspectives on ecological mechanisms affecting coral recruitment on reefs[J]. Smithsonian Contributions to the Marine Sciences, 2009, 38: 437−457. [14] Harrison P L. Sexual reproduction of scleractinian corals[M]//Dubinsky Z, Stambler N. Coral Reefs: An Ecosystem in Transition. Dordrecht: Springer, 2011: 59−85. [15] Edmunds P, Gates R, Gleason D. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances[J]. Marine Biology, 2001, 139(5): 981−989. doi: 10.1007/s002270100634 [16] Hughes T P, Tanner J E. Recruitment failure, life histories, and long-term decline of Caribbean corals[J]. Ecology, 2000, 81(8): 2250−2263. doi: 10.1890/0012-9658(2000)081[2250:RFLHAL]2.0.CO;2 [17] Richmond R H. Reproduction and recruitment in corals: critical links in the persistence of reefs[M]//Birkeland C. Life and Death of Coral Reefs. New York: Chapman and Hall, 1997. [18] Harrison P L, Wallace C C. Reproduction, dispersal and recruitment of scleractinian corals[M]//Dubinsky Z. Coral Reefs Ecosystems. Amsterdam: Elsevier, 1990: 133−207. [19] Randall C J, Szmant A M. Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper)[J]. Coral Reefs, 2009, 28(2): 537−545. [20] Hillyer K E, Dias D A, Lutz A, et al. Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera[J]. Coral Reefs, 2017, 36(1): 105−118. doi: 10.1007/s00338-016-1508-y [21] Nozawa Y, Harrison P L. Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis[J]. Marine Biology, 2007, 152(5): 1181−1185. doi: 10.1007/s00227-007-0765-2 [22] Jiang Lei, Sun Youfang, Zhang Yuyang, et al. Impact of diurnal temperature fluctuations on larval settlement and growth of the reef coral Pocillopora damicornis[J]. Biogeosciences, 2017, 14(24): 5741−5752. doi: 10.5194/bg-14-5741-2017 [23] Wall C B, Fan T Y, Edmunds P J. Ocean acidification has no effect on thermal bleaching in the coral Seriatopora caliendrum[J]. Coral Reefs, 2014, 33(1): 119−130. doi: 10.1007/s00338-013-1085-2 [24] Albright R, Mason B, Miller M, et al. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(47): 20400−20404. doi: 10.1073/pnas.1007273107 [25] Doropoulos C, Diaz-Pulido G. High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae[J]. Marine Ecology Progress Series, 2013, 475: 93−99. doi: 10.3354/meps10096 [26] Albright R, Langdon C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides[J]. Global Change Biology, 2011, 17(7): 2478−2487. doi: 10.1111/j.1365-2486.2011.02404.x [27] Foster T, Gilmour J P, Chua C M, et al. Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera[J]. Coral Reefs, 2015, 34(4): 1217−1226. doi: 10.1007/s00338-015-1342-7 [28] Webster N S, Webb R I, Ridd M J, et al. The effects of copper on the microbial community of a coral reef sponge[J]. Environmental Microbiology, 2001, 3(1): 19−31. doi: 10.1046/j.1462-2920.2001.00155.x [29] Tebben J, Tapiolas D M, Motti C A, et al. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium[J]. PLoS One, 2011, 6(4): e19082. doi: 10.1371/journal.pone.0019082 [30] Heyward A J, Negri A P. Natural inducers for coral larval metamorphosis[J]. Coral Reefs, 1999, 18(3): 273−279. doi: 10.1007/s003380050193 [31] Siboni N, Abrego D, Motti C A, et al. Gene expression patterns during the early stages of chemically induced larval metamorphosis and settlement of the coral Acropora millepora[J]. PLoS One, 2014, 9(3): e91082. doi: 10.1371/journal.pone.0091082 [32] Li Xiubao, Liu Sheng, Huang Hui, et al. Coral bleaching caused by an abnormal water temperature rise at Luhuitou fringing reef, Sanya Bay, China[J]. Aquatic Ecosystem Health & Management, 2012, 15(2): 227−233. [33] Baird A H, Gilmour J P, Kamiki T M, et al. Temperature tolerance of symbiotic and non-symbiotic coral larvae[C]//Proceedings of the 10th International Coral Reef Symposium. Okinawa, Japan: ICRS, 2006. [34] Yakovleva I, Baird A H, Yamamoto H H, et al. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures[J]. Marine Ecology Progress Series, 2009, 378: 105−112. doi: 10.3354/meps07857 [35] 江雷, 黄晖, 张浴阳, 等. 海水升温对壮实鹿角珊瑚幼虫存活和附着的影响[J]. 应用海洋学学报, 2016, 35(2): 217−222. doi: 10.3969/J.ISSN.2095-4972.2016.02.010Jiang Lei, Huang Hui, Zhang Yuyang, et al. Effects of elevated temperature on larval survival and settlement of the broadcast spawning coral Acropora robust[J]. Journal of Applied Oceanography, 2016, 35(2): 217−222. doi: 10.3969/J.ISSN.2095-4972.2016.02.010 [36] Olsen K, Ritson-Williams R, Paul V J, et al. Combined effects of macroalgal presence and elevated temperature on the early life-history stages of a common Caribbean coral[J]. Marine Ecology Progress Series, 2014, 509: 181−191. doi: 10.3354/meps10880 [37] Viyakarn V, Lalitpattarakit W, Chinfak N, et al. Effect of lower pH on settlement and development of coral, Pocillopora damicornis (Linnaeus, 1758)[J]. Ocean Science Journal, 2015, 50(2): 475−480. doi: 10.1007/s12601-015-0043-z [38] Suwa R, Nakamura M, Morita M, et al. Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora)[J]. Fisheries Science, 2010, 76(1): 93−99. doi: 10.1007/s12562-009-0189-7 [39] Putnam H M, Mayfield A B, Fan T Y, et al. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2[J]. Marine Biology, 2013, 160(8): 2157−2173. doi: 10.1007/s00227-012-2129-9 [40] Cumbo V R, Fan T Y, Edmunds P J. Effects of exposure duration on the response of Pocillopora damicornis larvae to elevated temperature and high pCO2[J]. Journal of Experimental Marine Biology and Ecology, 2013, 439: 100−107. doi: 10.1016/j.jembe.2012.10.019 [41] Marshall D J. Transgenerational plasticity in the sea: context-dependent maternal effects across the life history[J]. Ecology, 2008, 89(2): 418−427. doi: 10.1890/07-0449.1 [42] Jiang Lei, Huang Hui, Yuan Xiangcheng, et al. Effects of elevated pCO2 on the post-settlement development of Pocillopora damicornis[J]. Journal of Experimental Marine Biology and Ecology, 2015, 473: 169−175. doi: 10.1016/j.jembe.2015.09.004 [43] Jiang Lei, Zhang Fang, Guo Minglan, et al. Increased temperature mitigates the effects of ocean acidification on the calcification of juvenile Pocillopora damicornis, but at a cost[J]. Coral Reefs, 2018, 37(1): 71−79. doi: 10.1007/s00338-017-1634-1 [44] Anlauf H, D'Croz L, O'Dea A. A corrosive concoction: the combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative[J]. Journal of Experimental Marine Biology and Ecology, 2011, 397(1): 13−20. doi: 10.1016/j.jembe.2010.11.009 [45] Cohen A L, McCorkle D C, de Putron S, et al. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification[J]. Geochemistry, Geophysics, Geosystems, 2009, 10(7): Q07005. [46] Albright R, Mason B, Langdon C. Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae[J]. Coral Reefs, 2008, 27(3): 485−490. doi: 10.1007/s00338-008-0392-5 [47] Vermeij M J A, Sandin S A. Density-dependent settlement and mortality structure the earliest life phases of a coral population[J]. Ecology, 2008, 89(7): 1994−2004. doi: 10.1890/07-1296.1 [48] Hughes T P, Jackson J B C. Population dynamics and life histories of foliaceous corals[J]. Ecological Monographs, 1985, 55(2): 141−166. doi: 10.2307/1942555 [49] Yuan Xiangcheng, Yuan Tao, Huang Hui, et al. Elevated CO2 delays the early development of scleractinian coral Acropora gemmifera[J]. Scientific Reports, 2018, 8(1): 2787. doi: 10.1038/s41598-018-21267-3 [50] Moya A, Huisman L, Ball E E, et al. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification[J]. Molecular Ecology, 2012, 21(10): 2440−2454. doi: 10.1111/j.1365-294X.2012.05554.x -




 下载:
下载:

