Embryonic and larval early development of Favia favus and Platygyra carnosus in the Weizhou Island, Guangxi
-
摘要: 研究造礁石珊瑚的有性繁殖来探索珊瑚礁的生态修复是近年来的热点。本文于2018年5月采集广西涠洲岛自然海域中的黄癣蜂巢珊瑚(Favia favus)和肉质扁脑珊瑚(Platygyra carnosus)至室内养殖,收集受精卵,观察和记录其胚胎和幼虫的早期发育过程。结果显示,黄癣蜂巢珊瑚和肉质扁脑珊瑚都是雌雄同体,体外受精,在月圆后5~8 d产卵;发育过程都经历卵裂期、囊胚期、原肠胚期以及浮浪幼虫期;两者的卵母细胞都不含虫黄藻。本研究记录了涠洲岛造礁石珊瑚的有性繁殖行为,为进一步利用有性繁殖来进行珊瑚礁的生态修复提供了理论基础。Abstract: Sexual reproduction of hermatypic corals for coral reef restoration has become a hot topic in recent years. In May 2018, the samples of Favia favus and Platygyra carnosus were collected from the water of Weizhou Island, Guangxi. All of them were cultured in laboratory and the fertilized eggs were collected. The early development process of embryos and larvae was observed and recorded. The results showed that F.favus and P.carnosus were both hermaphrodites, in vitro fertilization, and began to spawn on the fifth to eighth day after the full moon; the early development of embryos and larvae experienced the cleavage stage, blastula stage, gastrula stage and planula stage; the oocytes of two corals did not have zooxanthella as they were ejected. This study records the sexual reproduction behavior of reef-building corals in Weizhou Island, and provides a theory when using sexual reproduction for coral ecological restoration in the future.
-
Key words:
- Favia favus /
- Platygyra carnosus /
- embryo and larvae /
- early development /
- sexual reproduction /
- Weizhou Island
-
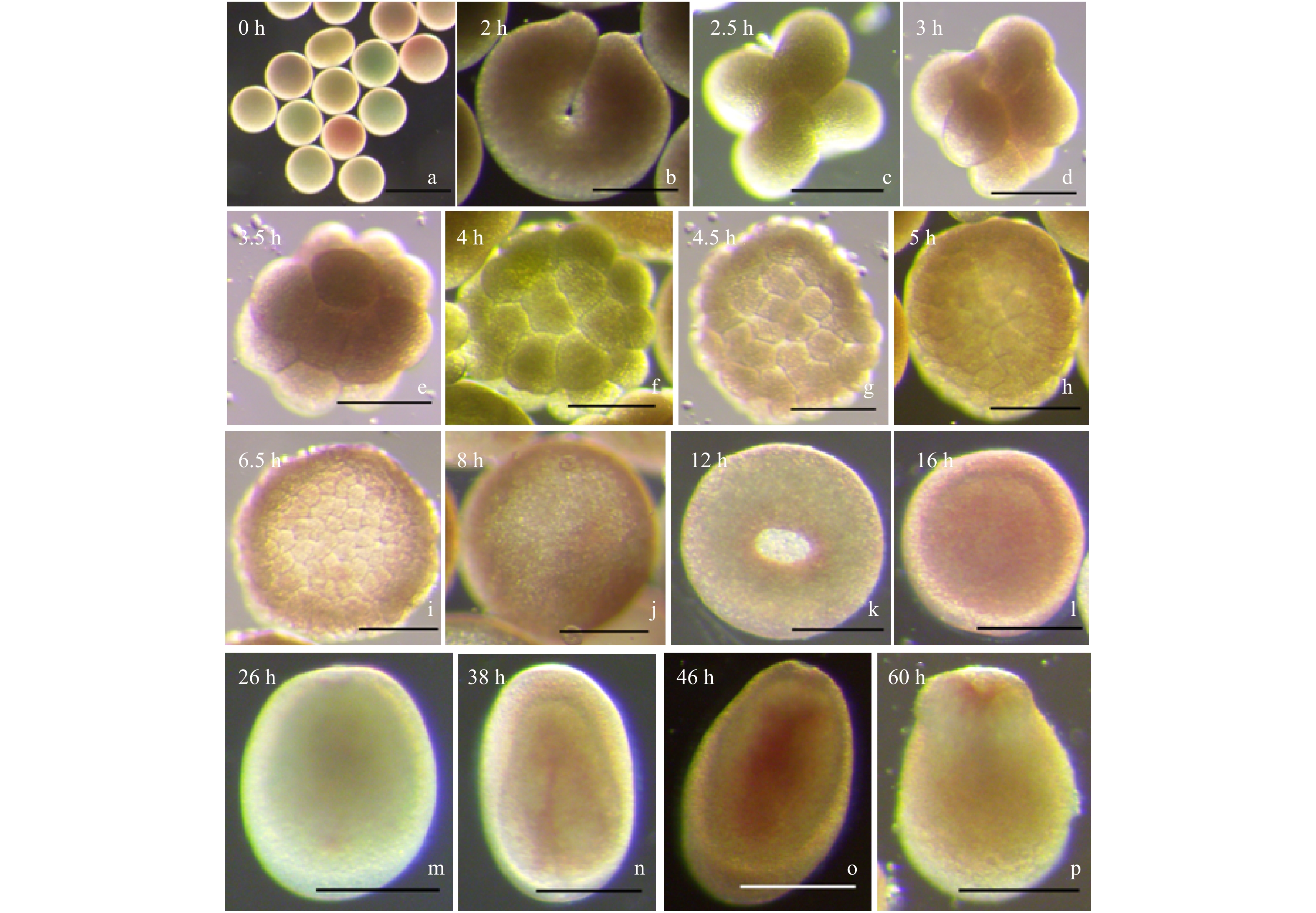
图 3 黄癣蜂巢珊瑚的早期发育过程
a. 卵母细胞;b. 2细胞期;c. 4细胞期;d. 8细胞期;e. 16细胞期;f. 32细胞期;g. 桑葚胚期(64细胞期);h. 桑葚胚期(128细胞期);i. 囊胚早期;j. 囊胚晚期;k. 原肠胚中期;l. 原肠胚晚期;m. 浮浪幼虫早期;n. 浮浪幼虫中期;o. 浮浪幼虫晚期;p. 浮浪幼虫末期. 除了标尺a为500 μm外,其余均为200 μm
Fig. 3 The early development of F. favus
a. Oocytes; b. 2-cell stage; c. 4-cell stage; d. 8-cell stage; e. 16-cell stage; f. 32-cell stage; g. morula stage (64-cell stage); h. morula stage (128-cell stage); i. early blastula stage; j. later blastula stage; k. middle gastrula stage; l. later gastrula stage; m. early planula stage; n. middle planula stage; o. later planula stage; p. the end of planula stage. All rulers indicate 200 μm besides Fig.3a shows 500 μm
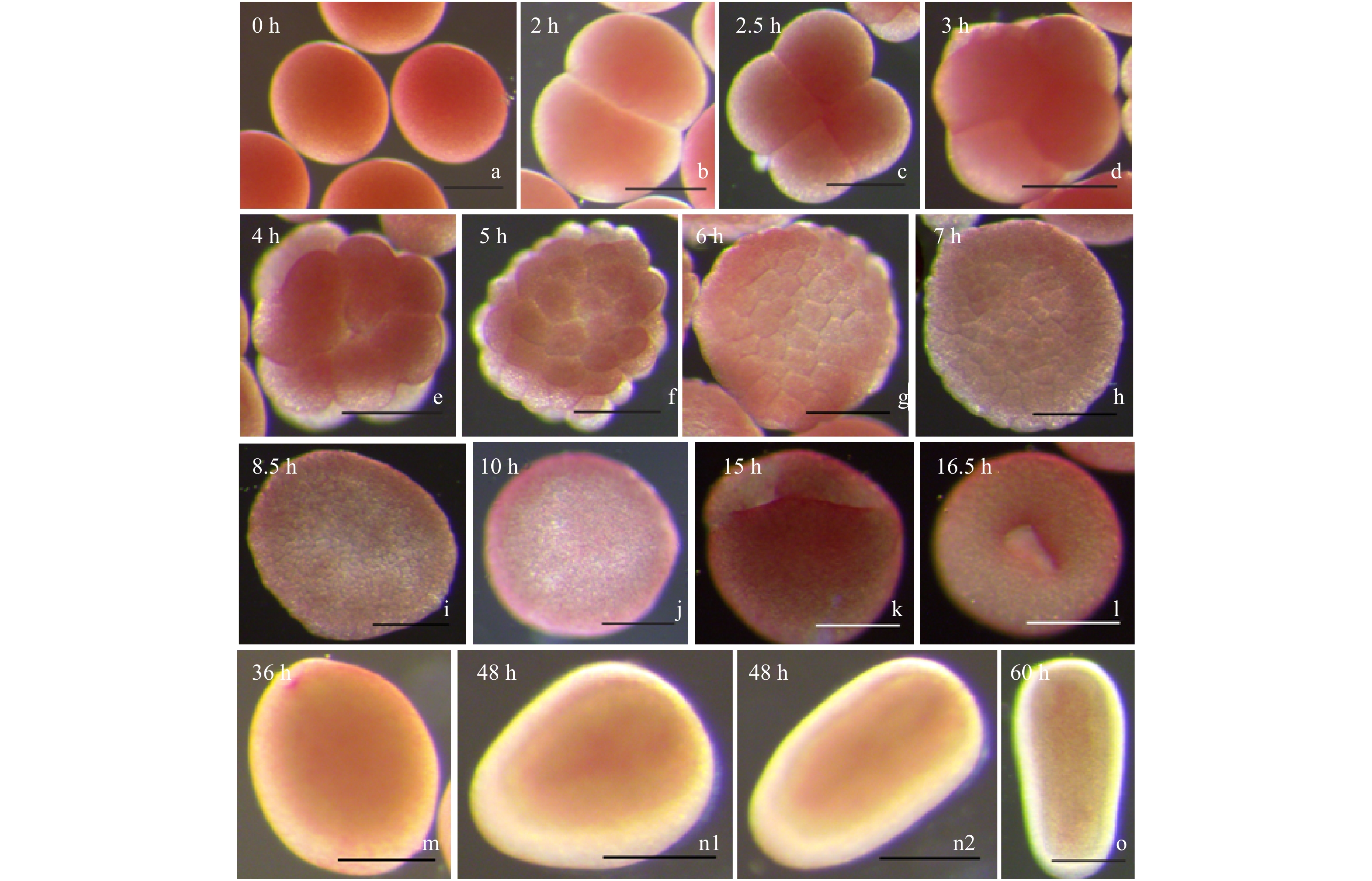
图 4 肉质扁脑珊瑚的早期发育过程
a. 卵母细胞;b. 2细胞期;c. 4细胞期;d. 8细胞期;e. 16细胞期;f. 32细胞期;g. 桑葚胚期(64细胞期);h. 桑葚胚期(128细胞期);i. 囊胚早期;j. 囊胚晚期;k. 原肠胚早期;l. 原肠胚中期;m. 浮浪幼虫早期;n1, n2. 浮浪幼虫中期;o. 浮浪幼虫晚期。所有标尺都为200 μm
Fig. 4 The early development of P.carnosus
a. Oocytes; b. 2-cell stage; c. 4-cell stage; d. 8-cell stage; e. 16-cell stage; f. 32-cell stage; g. morula stage (64-cell stage); h. morula stage (128-cell stage); i. early blastula stage; j. later blastula stage; k. early gastrula stage; l. middle gastrula stage; m. early planula stage; n1, n2. middle planula stage; o. later planula stage. All rulers indicate 200 μm
表 1 黄癣蜂巢珊瑚和肉质扁脑珊瑚的早期发育过程
Tab. 1 The early development of F.favus and P.carnosus
胚胎发育时期 黄癣蜂巢珊瑚 肉质扁脑珊瑚 受精后时间/h 发育特征 图示 受精后时间/h 发育特征 图示 卵母细胞 0 呈淡黄色、淡红色及淡青色,圆形 图3a 0 呈粉红色,圆形 图4a 2 细胞期 2.0 受精卵分裂成2个细胞 图3b 2.0 受精卵分裂成2个细胞 图4b 4 细胞期 2.5 受精卵分裂成4个细胞 图3c 2.5 受精卵分裂成4个细胞 图4c 8 细胞期 3.0 受精卵分裂成8个细胞 图3d 3.0 受精卵分裂成8个细胞 图4d 16 细胞期 3.5 受精卵分裂成16个细胞 图3e 4.0 受精卵分裂成16个细胞 图4e 32 细胞期 4.0 受精卵分裂成32个细胞 图3f 5.0 受精卵分裂成32个细胞,胚胎表面不规则 图4f 64 细胞期 4.5 受精卵分裂成64个细胞,形如桑葚 图3g 6.0 受精卵分裂成64个细胞,形如桑葚 图4g 128细胞期 5.0 受精卵分裂成128个细胞,形如桑葚 图3h 7.0 受精卵分裂成128个细胞,形如桑葚 图4h 囊胚早期 6.5 受精卵进一步分裂成128个细胞以上,胚胎中央透光明显 图3i 8.5 受精卵进一步分裂成128个细胞以上 图4i 囊胚中期 — — — — — — 囊胚晚期 8.0 胚胎表面开始变成平滑 图3j 10.0 胚胎表面变成平滑 图4j 原肠胚早期 — — — 15.0 胚胎内陷形成两个胚孔 图4k 原肠胚中期 12.0 出现胚孔,胚孔逐渐内陷形成口咽 图3k 16.5 两个胚孔融合成一个胚孔 图4l 原肠胚晚期 16.0 胚胎圆形,开始出现两个胚层的分化 图3l — — — 浮浪幼虫早期 26.0 浮浪幼虫成椭圆形,有一层明显的囊,转圈游动 图3m 36.0 胚孔逐渐闭合形成原口,开始出现两个胚层,漫无目的地轻轻游动 图4m 浮浪幼虫中期 38.0 浮浪幼虫呈梨形状或圆柱形,伸缩变形缓慢游动 图3n 48.0 胚胎成梨形或椭圆形,能够伸缩变形,四处游动 图4n1,图4n2 浮浪幼虫晚期 46.0 浮浪幼虫四处游动,两个胚层更加明显 图3o 60.0 出现肠系膜,胚层分化明显,变形游动 图4o 浮浪幼虫末期 60.0 出现了原口,随时准备附着 图3p — — — 注:—表示无数据。 -
[1] Moberg F, Folke C. Ecological goods and services of coral reef ecosystems[J]. Ecological Economics, 1999, 29(2): 215−233. doi: 10.1016/S0921-8009(99)00009-9 [2] Hughes T P, Baird A H, Bellwood D R, et al. Climate change, human impacts, and the resilience of coral reefs[J]. Science, 2003, 301(5635): 929−933. doi: 10.1126/science.1085046 [3] Hoegh-Guldberg O, Mumby P J, Hooten A J, et al. Coral reefs under rapid climate change and ocean acidification[J]. Science, 2007, 318(5857): 1737−1742. doi: 10.1126/science.1152509 [4] Carpenter K E, Abrar M, Aeby G, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts[J]. Science, 2008, 321(5888): 560−563. doi: 10.1126/science.1159196 [5] Edwards A J. Reef Rehabilitation Manual[M]. St Lucia, Australia: The Coral Reef Targeted Research & Capacity Building for Management Program, 2010: 1-166. [6] Gleason D F, Hofmann D K. Coral larvae: from gametes to recruits[J]. Journal of Experimental Marine Biology and Ecology, 2011, 408(1/2): 42−57. [7] Baird A H, Guest J R, Willis B L. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals[J]. Annual Review of Ecology, Evolution, and Systematics, 2009, 40: 551−571. doi: 10.1146/annurev.ecolsys.110308.120220 [8] Ayre D J, Hughes T P. Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia[J]. Evolution, 2000, 54(5): 1590−1605. doi: 10.1111/j.0014-3820.2000.tb00704.x [9] Harrison P L, Babcock R C, Bull G D, et al. Mass spawning in tropical reef corals[J]. Science, 1984, 223(4641): 1186−1189. doi: 10.1126/science.223.4641.1186 [10] Babcock R C, Bull G D, Harrison P L, et al. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef[J]. Marine Biology, 1986, 90(3): 379−394. doi: 10.1007/BF00428562 [11] Shlesinger Y, Goulet T L, Loya Y. Reproductive patterns of scleractinian corals in the northern Red Sea[J]. Marine Biology, 1998, 132(4): 691−701. doi: 10.1007/s002270050433 [12] Guest J R. Reproductive patterns of scleractinian corals on Singapore's reefs[D]. Singapore: National University of Singapore, 2004. [13] Nozawa Y, Tokeshi M, Nojima S. Reproduction and recruitment of scleractinian corals in a high-latitude coral community, Amakusa, southwestern Japan[J]. Marine Biology, 2006, 149(5): 1047−1058. doi: 10.1007/s00227-006-0285-5 [14] Okubo N, Motokawa T. Embryogenesis in the reef-building coral Acropora spp[J]. Zoological Science, 2007, 24(12): 1169−1178. doi: 10.2108/zsj.24.1169 [15] Mangubhai S, Harrison P L. Asynchronous coral spawning patterns on equatorial reefs in Kenya[J]. Marine Ecology Progress Series, 2008, 360: 85−96. doi: 10.3354/meps07385 [16] Kongjandtre N, Ridgway T, Ward S, et al. Broadcast spawning patterns of Favia species on the inshore reefs of Thailand[J]. Coral Reefs, 2010, 29(1): 227−234. doi: 10.1007/s00338-009-0551-3 [17] Chui A P Y, Wong M C, Liu S H, et al. Gametogenesis, embryogenesis, and fertilization ecology of Platygyra acuta in marginal nonreefal coral communities in Hong Kong[J]. Journal of Marine Biology, 2014, 2014: 953587. [18] 黄洁英, 黄晖, 张浴阳, 等. 膨胀蔷薇珊瑚与壮实鹿角珊瑚的胚胎和幼虫发育[J]. 热带海洋学报, 2011, 30(2): 67−73. doi: 10.3969/j.issn.1009-5470.2011.02.010Huang Jieying, Huang Hui, Zhang Yuyang, et al. Embryonic and larval development of Montipora turgescens and Acropora robusta[J]. Journal of Tropical Oceanography, 2011, 30(2): 67−73. doi: 10.3969/j.issn.1009-5470.2011.02.010 [19] 黄洁英. 三亚鹿回头海域造礁石珊瑚的有性繁殖生物学研究[D]. 北京: 中国科学院大学, 2011.Huang Jieying. The sexual reproductive biology of hermatypic corals in Luhuitou, Sanya, China[D]. Beijing: University of Chinese Academy of Sciences, 2011. [20] 张诗泽. 三亚鹿回头四种产卵型珊瑚有性繁殖生物学研究[D]. 北京: 中国科学院大学, 2014.Zhang Shize. The sexual reproductive biology of four spawning corals from Sanya Luhuitou of Hainan Island[D]. Beijing: University of Chinese Academy of Sciences, 2014. [21] 张浴阳, 黄晖, 黄洁英, 等. 西沙群岛珊瑚幼体培育实验[J]. 海洋开发与管理, 2013, 30(S1): 78−82.Zhang Yuyang, Huang Hui, Huang Jieying, et al. The breeding test of coral larvae in Xisha Islands[J]. Ocean Development and Management, 2013, 30(S1): 78−82. [22] 朱潜, 孙杨, 肖业有, 等. 风信子鹿角珊瑚的胚胎和幼虫发育研究[J]. 大连海洋大学学报, 2014, 29(5): 444−448. doi: 10.3969/J.ISSN.2095-1388.2014.05.004Zhu Qian, Sun Yang, Xiao Yeyou, et al. Embryonic and larval development of coral Acropora hyacinthus[J]. Journal of Dalian Ocean University, 2014, 29(5): 444−448. doi: 10.3969/J.ISSN.2095-1388.2014.05.004 [23] 肖宝华, 廖宝林, 杨小东, 等. 肉质扁脑珊瑚的有性繁殖及早期发育[J]. 热带海洋学报, 2017, 36(1): 65−71.Xiao Baohua, Liao Baolin, Yang Xiaodong, et al. Sexual reproduction and early development of Platygyra carnosus[J]. Journal of Tropical Oceanography, 2017, 36(1): 65−71. [24] 邹仁林. 中国动物志, 腔肠动物门, 珊瑚虫纲, 石珊瑚目, 造礁石珊瑚[M]. 北京: 科学出版社, 2001: 1-289.Zou Renlin. Fauna Sinica, Coelenterata, Anthozoa, Scleratinia, Scleractinian Corals[M]. Beijing: Science Press, 2001: 1−289. [25] 王文欢. 近30年来北部湾涠洲岛造礁石珊瑚群落演变及影响因素[D]. 南宁: 广西大学, 2017.Wang Wenhuan. Evolvement and influential factors of coral community over past three decases in Weizhou Island reef, Beibu Gulf[D]. Nanning: Guangxi University, 2017. [26] Rinkevich B, Loya Y. The reproduction of the Red Sea coral Stylophora pistillata. Ⅰ. Gonads and planulae[J]. Marine Ecology Progress Series, 1979, 1: 133−144. doi: 10.3354/meps001133 [27] Harriott V J. Reproductive ecology of four scleratinian species at Lizard Island, Great Barrier Reef[J]. Coral Reefs, 1983, 2(1): 9−18. doi: 10.1007/BF00304727 [28] Shlesinger Y, Loya Y. Larval development and survivorship in the corals Favia favus and Platygyra lamellina[J]. Hydrobiologia, 1991, 216(1): 101−108. [29] Oliver J, Babcock R. Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization[J]. The Biological Bulletin, 1992, 183(3): 409−417. doi: 10.2307/1542017 [30] Randall C J, Szmant A M. Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816)[J]. The Biological Bulletin, 2009, 217(3): 269−282. doi: 10.1086/BBLv217n3p269 [31] Baums I B, Devlin-Durante M K, Polato N R, et al. Genotypic variation influences reproductive success and thermal stress tolerance in the reef building coral, Acropora palmata[J]. Coral Reefs, 2013, 32(3): 703−717. doi: 10.1007/s00338-013-1012-6 [32] Babcock R C, Heyward A J. Larval development of certain gamete-spawning scleractinian corals[J]. Coral Reefs, 1986, 5(3): 111−116. doi: 10.1007/BF00298178 [33] Okubo N, Mezaki T, Nozawa Y, et al. Comparative embryology of eleven species of stony corals (Scleractinia)[J]. PLoS One, 2013, 8(12): e84115. doi: 10.1371/journal.pone.0084115 [34] Hirose M, Kinzie R, Hidaka M. Timing and process of entry of zooxanthellae into oocytes of hermatypic corals[J]. Coral Reefs, 2001, 20(3): 273−280. doi: 10.1007/s003380100171 [35] Yakovleva I M, Baird A H, Yamamoto H H, et al. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures[J]. Marine Ecology Progress Series, 2009, 378: 105−112. doi: 10.3354/meps07857 [36] Negri A P, Marshall P A, Heyward A J. Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species[J]. Coral Reefs, 2007, 26(4): 759−763. doi: 10.1007/s00338-007-0258-2 [37] Jones R, Ricardo G F, Negri A P. Effects of sediments on the reproductive cycle of corals[J]. Marine Pollution Bulletin, 2015, 100(1): 13−33. doi: 10.1016/j.marpolbul.2015.08.021 -





 下载:
下载: