缢蛏(Sinonovacula constricta)GST和HSP90基因克隆及其在氨氮胁迫下的表达特征分析
doi: 10.3969/j.issn.0253-4193.2020.04.008
Cloning of GST and HSP90 genes of Sinonovacula constricta and analysis of their expression characteristics under ammonia nitrogen stress
-
摘要: 克隆获得缢蛏(Sinonovacula constricta)谷胱甘肽S-转移酶(Sc-GSTσ)和热休克蛋白90(Sc-HSP90)基因的cDNA全长,分析了它们的组织表达差异及其在氨氮胁迫下的表达特征。结果表明,Sc-GSTσ的全长cDNA为1 414 bp,含有639 bp的开放阅读框(Open Reading Frame,ORF),编码212个氨基酸,Sc-GSTσ氨基酸序列与其他物种的GST氨基酸序列同源性为31.88%~43.40%;而Sc-HSP90的全长cDNA为2 752 bp,ORF为2 181 bp,编码726个氨基酸,其氨基酸序列与其他物种HSP90的氨基酸序列同源性为76.77%~87.05%。荧光定量PCR分析发现,Sc-GSTσ和Sc-HSP90在缢蛏各组织中均有表达,两者均在肝胰腺中表达量最高。氨氮胁迫后,Sc-GSTσ和Sc-HSP90 mRNA在肝胰腺中表达均显著上调(p<0.05),表明氨氮胁迫引起机体的应激反应,2个基因可能参与机体解毒或防御过程。但胁迫后期表达量下降推测是机体的防御能力有限,不足以完全保护宿主免受应激诱导的细胞损伤。Abstract: The full-length cDNA of glutathione S-transferase (Sc-GSTσ) and heat shock protein 90 (Sc-HSP90) genes were cloned from Sinonovacula constricta and their expression characteristics under ammonia nitrogen stress were analyzed. The results show that the full-length cDNA of Sc-GSTσ was 1 414 bp, and containing 639 bp open reading frame (ORF), encoding 212 amino acid polypeptides. The homology of amino acid sequence of Sc-GSTσ with other species’ GST amino acid sequence was 31.88%−43.40%. The full-length cDNA of Sc-HSP90 was 2 752 bp, ORF was 2 181 bp, encoding 726 amino acids. The amino acid sequence was 76.77%−87.05% homology with other species. Quantitative analysis showed that Sc-GSTσ and Sc-HSP90 genes were expressed in all tested tissues, the strongest expression being in the digestive gland. After exposure to ammonia, the mRNA expression of Sc-GSTσ and Sc-HSP90 were significantly up-regulated (p<0.05) in the digestive gland, indicating that ammonia stress induced stress response, both GST and HSP90 may be participate the process of detoxification or defense. However, the decrease of expression in the later period of stress is presumed to be due to the organism have limited ability to defense, which is not enough to protect the host from stress-induced cell damage.
-
Key words:
- Sinonovacula constricta /
- GST /
- HSP90 /
- ammonia nitrogen /
- expression characteristics
-
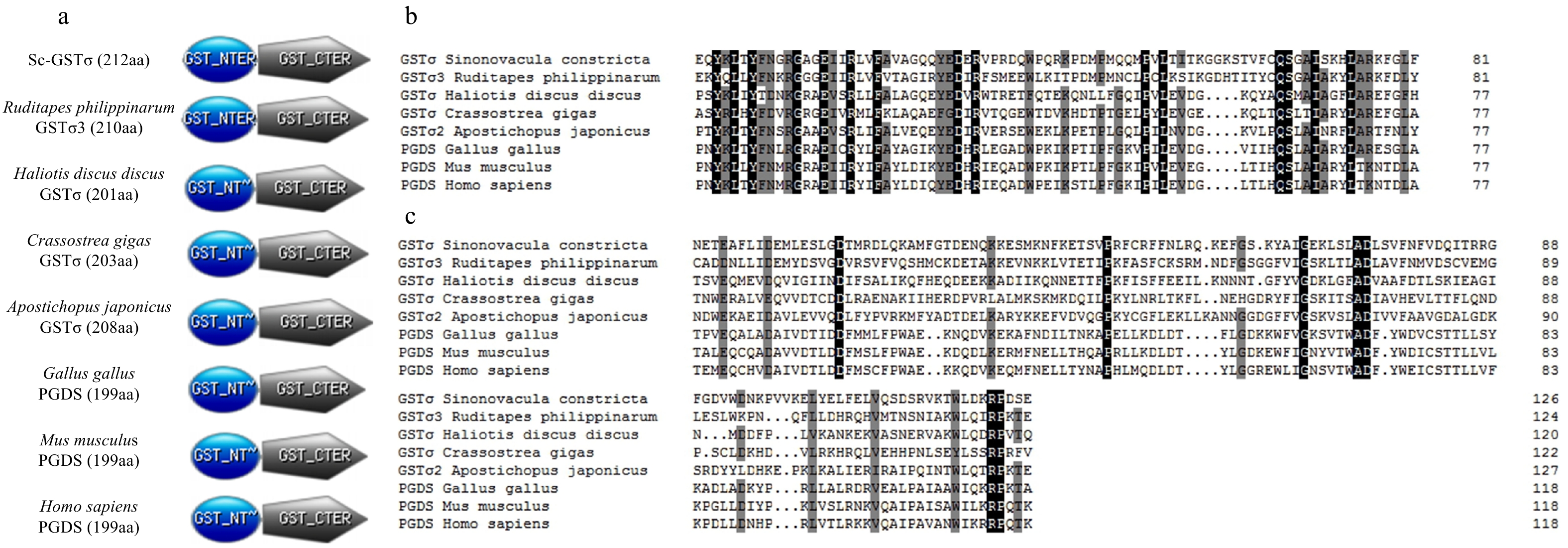
图 1 Sc-GSTσ结构域和多重比较
a. Sc-GSTσ的结构域与挑选的4种无脊椎动物的GSTσ和3种脊椎动物的前列腺素D合成酶(PGD,A GSTσ)结构域比较;b. 8种动物N末端结构域多重比较;c. 8种动物GSTσ C末端结构域多重比较
Fig. 1 Sc-GSTσ domain structure and multiple sequence alignment
a. The structure domains of Sc-GSTσ were compared with those of four selected other invertebrate GSTs and three vertebrate prostaglandin-D synthases (PGDS, a GSTσ); b. multiple sequence alignment of N-terminal domain from eight animals; c. multiple sequence alignment of GSTσ C-terminal domain from eight animals
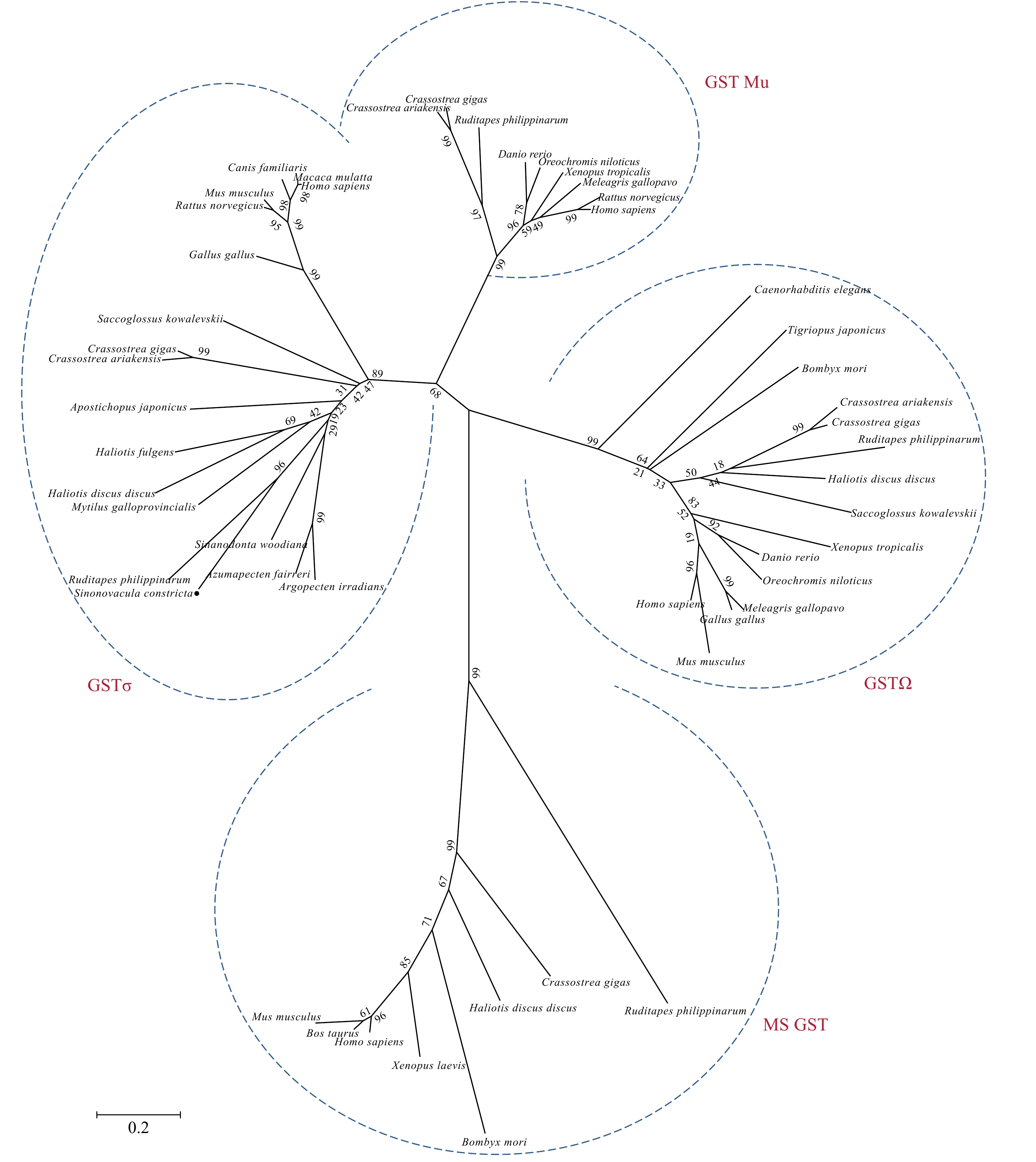
图 3 Sc-HSP90基因鉴定及系统进化树
a. 8种动物HSP90氨基酸序列多重比较,红色方框为HSP90家族标签,保守序列MEEVD带有下划线;b. 利用MEGA5.2软件采用邻接法构建的HSP90的系统进化树。使用序列的登录号见附录表A2
Fig. 3 Sc-HSP90 gene identified and phylogenetic tree
a. Multiple sequence alignment of HSP90 amino acid sequences from eight animals, HSP90 family signature motifs are red boxes and sequence MEEVD is underlined; b. the phylogenetic tree of HSP90 constructed by Neighbor-joining method using MEGA5.2 software. Accession numbers of the sequences used in construction of tree are attached in the appendix Table A2
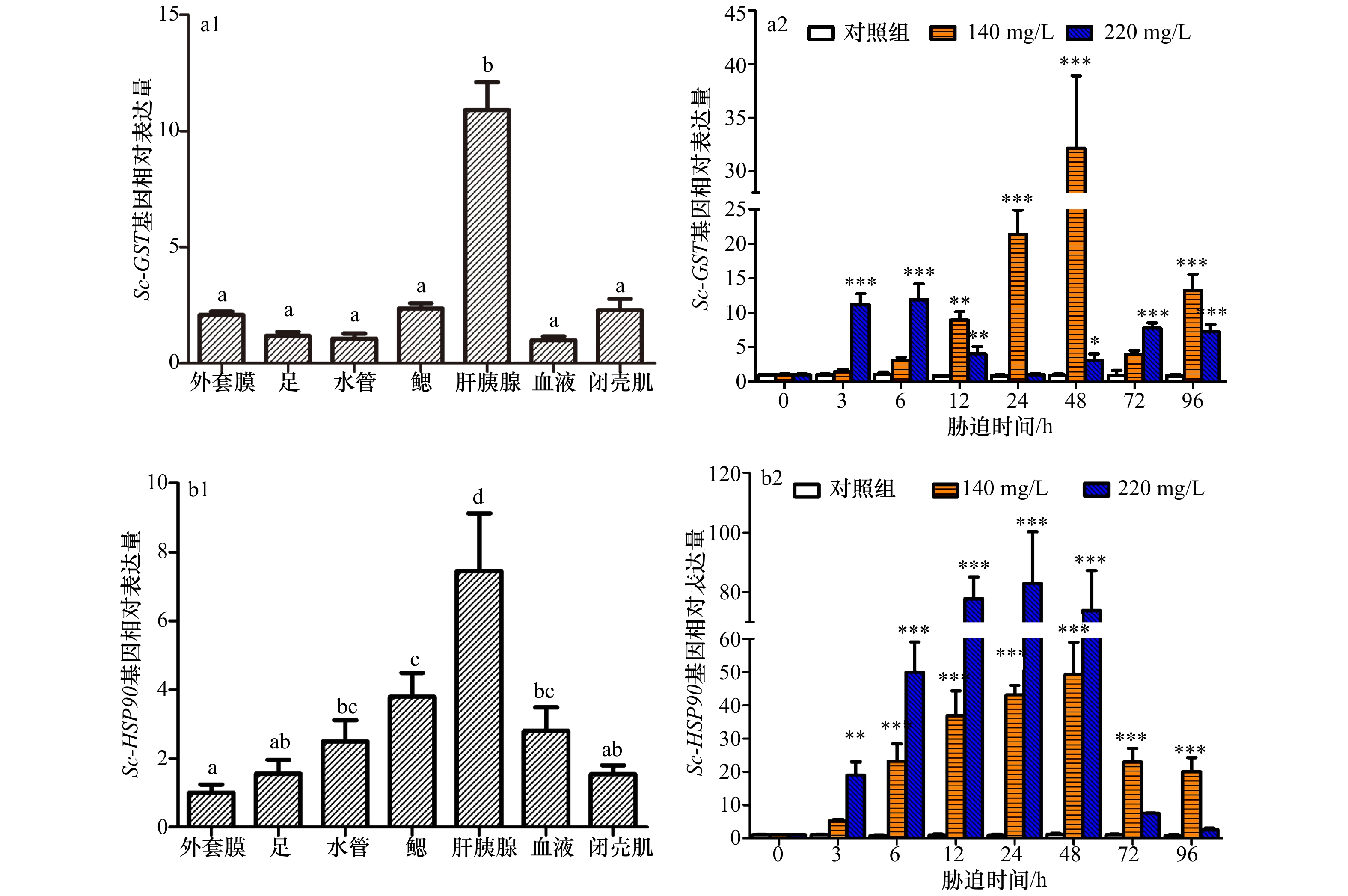
图 4 Sc-GSTσ和Sc-HSP90基因在缢蛏不同组织中及不同氨浓度胁迫后的表达特征
a1. Sc-GSTσ基因的组织表达;a2. 不同氨浓度胁迫后,Sc-GSTσ在缢蛏肝胰腺中的表达量变化;b1. Sc-HSP90基因的组织表达;b2. 不同氨浓度胁迫后,Sc-HSP90在缢蛏肝胰腺中的表达量变化。组织间不同字母的数据差异显著(p<0.05),星号(*)表示在各时间点胁迫组与对照组之间的显著差异(*p<0.05;**p<0.01;***p<0.001)
Fig. 4 The expression characteristics of Sc-GSTσ and Sc-HSP90 genes in different tissues of S. constricta and after different ammonia concentration stress
a1. Expression profile of Sc-GSTσ in S. constricta; a2. expression characteristic analysis of Sc-GSTσ in the digestive gland of S. constricta after ammonia stress; b1. expression profile of Sc-HSP90 in S. contricta; b2. expression characteristic analysis of Sc-HSP90 in the digestive gland of S. contricta data after ammonia stress. Different letters are significantly different among tissues (p<0.05) and the asterisk (*) indicates a significant difference between the stress group and its respective control at different time (*p<0.05; **p<0.01; ***p<0.001)
表 1 本实验所用引物及序列信息
Tab. 1 The information of primers and sequences used in this experiment
引物名称 序列(5′-3′) 引物信息 Long CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT RACE扩增接头引物 Short CTAATACGACTCACTATAGGGC RACE扩增接头引物 T3 GAGAGTGCTCGCAACTTAGAAATCGCCG Sc-GSTσ 3′RACE扩增 T5 CGGCATGTCTGGTTTCCGTTGG Sc-GSTσ 5′RACE扩增 P3 CGCATCCCTCAATACTCTTGGTCG Sc-HSP90 3′RACE扩增 P5 CCTCGTCTTCCTCCAAATCCTCTACCTT Sc-HSP90 5′RACE扩增 M13 F CGCCAGGGTTTTCCCAGTCACGAC T1载体通用引物 M13 R GAGCGGATAACAATTTCACACAGG T1载体通用引物 T F1 TGGAGTCTCTGGGTGATACG Sc-GSTσ全长验证引物 T R1 ACAACAACGCTGAAACTGGA Sc-GSTσ全长验证引物 T F2 TTCTTGTGGAGCAGTACAGTTCTAA Sc-GSTσ全长验证引物 T R2 CAAAATCAAAAGGCTGTCGC Sc-GSTσ全长验证引物 P F1 GTTTACTCAGAAAGACGCACGG Sc-HSP90全长验证引物 P R1 CTACTACGACGCATAATGGAGC Sc-HSP90全长验证引物 P F2 GGCTGCCAAGAAGAACCTA Sc-HSP90全长验证引物 P R2 AAATCACAGATTGAACAATCAAGTA Sc-HSP90全长验证引物 RS9 F TGAAGTCTGGCGTGTCAAGT 内参基因荧光定量引物 RS9 R CGTCTCAAAAGGGCATTACC 内参基因荧光定量引物 GST F GAACCGACGAGAATCAGAAGA Sc-GSTσ荧光定量引物 GST R AAACATCGCCGAAACCACG Sc-GSTσ荧光定量引物 HSP90 F GATGAAGGGGAGGTGGAAA Sc-HSP90荧光定量引物 HSP90 R GTGTCAATGAGGGTCAGTGTGT Sc-HSP90荧光定量引物 -
[1] 刘胜男. 三疣梭子蟹(Portunus trituberculatus)在氨氮胁迫下解毒代谢机制的研究[D]. 青岛: 中国海洋大学, 2014.Liu Shengnan. Study on detoxification metabolic mechanism of swimming crab Portunus trituberculatus exposed to ammonia[D]. Qingdao: Ocean University of China, 2014. [2] Ip Y K, Chew S F, Randall D J. Ammonia toxicity, tolerance, and excretion[J]. Fish Physiology, 2001, 20: 109−148. doi: 10.1016/S1546-5098(01)20005-3 [3] Miranda-Filho K C, Pinho G L L, Wasielesky W, et al. Long-term ammonia toxicity to the pink-shrimp Farfantepenaeus paulensis[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2009, 150(3): 377−382. [4] Cheng Changhong, Yang Fangfang, Ling Renzhi, et al. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus)[J]. Aquatic Toxicology, 2015, 164: 61−71. doi: 10.1016/j.aquatox.2015.04.004 [5] Zhang Lan, Pan Luqing, Xu Lijun, et al. Effects of ammonia-N exposure on the concentrations of neurotransmitters, hemocyte intracellular signaling pathways and immune responses in white shrimp Litopenaeus vannamei[J]. Fish & Shellfish Immunology, 2018, 75: 48−57. [6] Randall D J, Tsui T K N. Ammonia toxicity in fish[J]. Marine Pollution Bulletin, 2002, 45(1/12): 17−23. [7] Wilkinson D J, Smeeton N J, Watt P W. Ammonia metabolism, the brain and fatigue; revisiting the link[J]. Progress in Neurobiology, 2010, 91(3): 200−219. doi: 10.1016/j.pneurobio.2010.01.012 [8] Ren Qin, Pan Luqing. Digital gene expression analysis in the gills of the swimming crab (Portunus trituberculatus) exposed to elevated ambient ammonia-N[J]. Aquaculture, 2014, 434: 108−114. doi: 10.1016/j.aquaculture.2014.08.008 [9] Zhang Muzi, Li Ming, Wang Rixin, et al. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine[J]. Fish & Shellfish Immunology, 2018, 79: 313−320. [10] Zhu Limei, Gao N, Wang Ruifang, et al. Proteomic and metabolomic analysis of marine medaka (Oryzias melastigma) after acute ammonia exposure[J]. Ecotoxicology, 2018, 27(3): 267−277. doi: 10.1007/s10646-017-1892-2 [11] Qi Xiaozhou, Xue Mingyang, Yang Shibo, et al. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus)[J]. Fish & Shellfish Immunology, 2017, 70: 485−492. [12] Cheng Changhong, Guo Zhixun, Wang Anli. Growth performance and protective effect of vitamin E on oxidative stress pufferfish (Takifugu obscurus) following by ammonia stress[J]. Fish Physiology and Biochemistry, 2018, 44(2): 735−745. doi: 10.1007/s10695-018-0468-2 [13] Hegazi M M, Attia Z I, Ashour O A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure[J]. Aquatic Toxicology, 2010, 99(2): 118−125. doi: 10.1016/j.aquatox.2010.04.007 [14] Sinha A K, Zinta G, AbdElgawad H, et al. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax)[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2015, 174−175: 21−31. [15] Pinto M R, Lucena M N, Faleiros R O, et al. Effects of ammonia stress in the Amazon river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae)[J]. Aquatic Toxicology, 2016, 170: 13−23. doi: 10.1016/j.aquatox.2015.10.021 [16] Huang Jia, Wu Shunfan, Ye Gongyin. Molecular characterization of the sigma class gutathione S-transferase from Chilo suppressalis and expression analysis upon bacterial and insecticidal challenge[J]. Journal of Economic Entomology, 2011, 104(6): 2046−2053. doi: 10.1603/EC11181 [17] 刘慧慧, 何建瑜, 赵荣涛, 等. 重金属胁迫下厚壳贻贝谷胱甘肽S-转移酶基因表达分析[J]. 海洋与湖沼, 2014, 45(2): 274−280. doi: 10.11693/hyhz20121204002Liu Huihui, He Jiayu, Zhao Rongtao, et al. Molecular expression pattern of glutathione S-transferase gene in Mytilus coruscus exposed to heavy metals[J]. Oceanologia et Limnologia Sinica, 2014, 45(2): 274−280. doi: 10.11693/hyhz20121204002 [18] Costa S, Guilhermino L. Influence of long-term exposure to background pollution on the response and recovery of the invasive species Corbicula fluminea to ammonia sub-lethal stress: a multi-marker approach with field estuarine populations[J]. Water, Air, & Soil Pollution, 2015, 226(4): 95. [19] Li H, Yang Z, Huang Q, et al. Molecular cloning and characterization of a sigma-class glutathione S-Transferase from the freshwater mussel Hyriopsis cumingii[J]. Microbiology and Immunology, 2015, 59(4): 219−230. doi: 10.1111/1348-0421.12250 [20] Park H, Ahn I Y, Kim H, et al. Glutathione S-transferase as a biomarker in the Antarctic bivalve Laternula elliptica after exposure to the polychlorinated biphenyl mixture Aroclor 1254[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2009, 150(4): 528−536. [21] 孙盛明, 朱健, 戈贤平, 等. 团头鲂谷胱甘肽S-转移酶基因的克隆及其在氨氮胁迫中的表达分析[J]. 生态毒理学报, 2016, 11(1): 295−305.Sun Shengming, Zhu Jian, Ge Xianping, et al. Molecular cloning, characterization and mRNA expression of Mu-typ glutathione S-transferases from Megalobrama amblycephala[J]. Asian Journal of Ecotoxicology, 2016, 11(1): 295−305. [22] Qin Chuanjie, Shao Ting, Duan Huiguo. The cloning of a heat shock protein 90β gene and expression analysis in Botia reevesae after ammonia-N exposure and Aeromonas hydrophila challenge[J]. Aquaculture Report, 2016, 3: 159−165. doi: 10.1016/j.aqrep.2016.02.004 [23] Lu Xia, Luan Sheng, Dai Ping, et al. iTRAQ-based comparative proteome analysis for molecular mechanism of defense against acute ammonia toxicity in Pacific white shrimp Litopenaeus vannamei[J]. Fish & Shellfish Immunology, 2018, 74: 52−61. [24] Zhou Xin, Dong Yunwei, Wang Fang, et al. The effect of high ammonia concentration on gill structure alternation and expression of SOD and HSP90 genes in grass carp, Ctenopharyngodon idella[J]. Acta Hydrobiologica Sinica, 2013, 37(2): 321−328. [25] Li Jitao, Han Junying, Chen Ping, et al. Cloning of a heat shock protein 90 (HSP90) gene and expression analysis in the ridgetail white prawn Exopalaemon carinicauda[J]. Fish & Shellfish Immunology, 2012, 32(6): 1191−1197. [26] Wang Xingqiang, Wang Lingling, Yao Chen, et al. Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge[J]. Fish & Shellfish Immunology, 2012, 32(5): 741−749. [27] 农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2018中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2018.The Ministry of Agriculture and Fishery of the People's Republic of China, National Aquatic Technology Promotion Terminal, Chinese Society of Fisheries. Chinese Fishery Statistical Yearbook 2018[M]. Beijing: China Agriculture Press, 2018. [28] Guo Xiaoyu, Feng Chenghong. Biological toxicity response of Asian clam (Corbicula fluminea) to pollutants in surface water and sediment[J]. Science of the Total Environment, 2018, 631-632: 56−70. doi: 10.1016/j.scitotenv.2018.03.019 [29] Fu Jianping, Zhao Xuelin, Shi Yuhong, et al. Functional characterization of two ABC transporters in Sinonovacula constricta gills and their barrier action in response to pathogen infection[J]. International Journal of Biological Macromolecules, 2019, 121: 443−453. doi: 10.1016/j.ijbiomac.2018.10.047 [30] Zhao Xuelin, Fu Jianping, Jiang Liting, et al. Transcriptome-based identification of the optimal reference genes as internal controls for quantitative RT-PCR in razor clam (Sinonovacula constricta)[J]. Genes & Genomics, 2018, 40(6): 603−613. [31] 陈丽君. 谷胱甘肽S-转移酶基因家族的研究进展[J]. 皖南医学院学报, 2003, 22(2): 144−146. doi: 10.3969/j.issn.1002-0217.2003.02.029Chen Lijun. Research progress of glutathione S-transferase gene family[J]. Acta Academiae Medicinae Wannan, 2003, 22(2): 144−146. doi: 10.3969/j.issn.1002-0217.2003.02.029 [32] 程炜轩, 梁旭方, 李观贵, 等. 鳜鱼两种谷胱甘肽S-转移酶基因cDNA的克隆与分析[J]. 生态毒理学报, 2009, 4(4): 537−543.Cheng Weixuan, Liang Xufang, Li Guangui, et al. Molecular cloning and sequence analysis of alpha-and rho-classes of glutathione S-transferases in Chinese perch (Siniperca chuatsi)[J]. Asian Journal of Ecotoxicology, 2009, 4(4): 537−543. [33] 余作奔, 王春琳, 母昌考, 等. 曼氏无针乌贼(Sepiella maindroni)sigma-型谷胱甘肽硫转移酶基因的克隆及重组表达[J]. 海洋与湖沼, 2013, 44(2): 488−492. doi: 10.11693/hyhz201302035035Yu Zuoben, Wang Chunlin, Mu Changkao, et al. Molecular cloning and recombinant expression of a sigma-like glutathione S-transferase from Sepiella maindroni[J]. Oceanologia et Limnologia Sinica, 2013, 44(2): 488−492. doi: 10.11693/hyhz201302035035 [34] Umasuthan N, Revathy K S, Lee Y, et al. A novel molluscan sigma-like glutathione S-transferase from Manila clam, Ruditapes philippinarum: Cloning, characterization and transcriptional profiling[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2012, 155(4): 539−550. [35] 张飚, 李永清, 高轩. 谷胱甘肽S-转移酶综述[J]. 吉林畜牧兽医, 2006, 27(6): 11−13. doi: 10.3969/j.issn.1672-2078.2006.06.005Zhang Biao, Li Yongqing, Gao Xuan. Summarize of glutathione S-transferases[J]. Jilin Animal Science and Veterinary Medicine, 2006, 27(6): 11−13. doi: 10.3969/j.issn.1672-2078.2006.06.005 [36] 冯冰冰, 牛东红, 钟玉民, 等. 缢蛏ScHsc70 cDNA的分子特性和表达分析[J]. 中国水产科学, 2012, 19(1): 33−44.Feng Bingbing, Niu Donghong, Zhong Yumin, et al. Molecular characteristics and expression analysis of ScHsc70 cDNA in agamaki clam (Sinonovacula constricta)[J]. Journal of Fishery Sciences of China, 2012, 19(1): 33−44. [37] 冯冰冰. 缢蛏热休克蛋白Sc-sHSP基因的分子特性与表达分析[C]//2012年中国水产学会学术年会论文摘要集. 郑州: 中国水产学会, 2012.Feng Bingbing. Molecular characteristics and expression analysis of Sc-sHSP gene from Sinonovacula constricta[C]// Abstracts Book of Papers Presented at the 2012 Annual Academic Meeting of the Chinese Fisheries Society. Zhengzhou: China Society of Fisheries, 2012. [38] Prodromou C, Siligardi G, O'Brien R, et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones[J]. The EMBO Journal, 1999, 18(3): 754−762. doi: 10.1093/emboj/18.3.754 [39] Ding J, Wang H, Yin C, et al. Molecular cloning of the heat shock protein 90 gene in scallop Mizuhopecten yessoensis and the effects of temperature stress on gene expression[J]. Invertebrate Survival Journal, 2018, 15(1): 2−13. [40] Liang H Y, Wang Z X, Lei Q N, et al. Molecular cloning and expression analysis of a pearl oyster (Pinctada martensii) heat shock protein 90 (HSP90)[J]. Genetics and Molecular Research, 2015, 14(4): 18778−18791. doi: 10.4238/2015.December.28.27 [41] Sun S M, Zhu J, Ge X P, et al. Cloning and expression analysis of a heat shock protein 90β isoform gene from the gills of Wuchang bream (Megalobrama amblycephala Yih) subjected to nitrite stress[J]. Genetics and Molecular Research, 2015, 14(2): 3036−3051. doi: 10.4238/2015.April.10.14 [42] Ren Hai, Li Jian, Li Jitao, et al. Transcript profiles of mitochondrial and cytoplasmic manganese superoxide dismutases in Exopalaemon carinicauda under ammonia stress[J]. Chinese Journal of Oceanology and Limnology, 2015, 33(3): 714−724. doi: 10.1007/s00343-015-4143-5 [43] Li Ming, Chen Liqiao, Qin J G, et al. Growth performance, antioxidant status and immune response in darkbarbel catfish Pelteobagrus vachelli fed different PUFA/vitamin E dietary levels and exposed to high or low ammonia[J]. Aquaculture, 2013, 406-407: 18−27. doi: 10.1016/j.aquaculture.2013.04.028 [44] Hangzo H, Banerjee B, Saha S, et al. Ammonia stress under high environmental ammonia induces Hsp70 and Hsp90 in the mud eel, Monopterus cuchia[J]. Fish Physiology and Biochemistry, 2017, 43(1): 77−88. doi: 10.1007/s10695-016-0269-4 [45] Zhang Chunnuan, Li Xiangfei, Tian Hongyan, et al. Effects of fructooligosaccharide on immune response, antioxidant capability and HSP70 and HSP90 expressions of blunt snout bream (Megalobrama amblycephala) under high ammonia stress[J]. Fish Physiology and Biochemistry, 2015, 41(1): 203−217. doi: 10.1007/s10695-014-0017-6 -




 下载:
下载: