阿根廷巴塔哥尼亚陆架拉氏南美南极鱼(Patagonotothen ramsayi)不同组织中脂肪酸分布及食物来源指示
doi: 10.3969/j.issn.0253-4193.2020.04.003
The distribution of fatty acids in tissues of rock cod (Patagonotothen ramsayi) in the Argentina Patagonian Shelf and their implications to feeding habit
-
摘要: 作为南美南极鱼科中数量最为丰富的一个种,拉氏南美南极鱼(Patagonotothen ramsayi)在巴塔哥尼亚海域食物网能量流动中起着重要的传递作用。目前针对该鱼种的营养及摄食生态学研究主要集中于反映短期摄食的传统胃含物分析上。为此,本研究分析了拉氏南美南极鱼3种组织(肌肉、肝脏和性腺)中脂肪酸含量及分布情况,并就3种组织中的脂肪酸是否能表征其食性及食性转换进行了探究。结果表明,拉氏南美南极鱼体内共检测出27种脂肪酸;由于涉及生长、繁殖等因素影响,肝脏、性腺组织对于脂肪酸的储存及使用情况并不适用于指征食性,而肌肉组织更新时间相对较长,故能够较好地反映其对食物中脂肪酸的吸收。对不同体长组拉氏南美南极鱼肌肉组织中脂肪酸分析可知,未成熟拉氏南美南极鱼(100~240 mm)主要摄食浮游性生物;随着体长增加对底栖生物的摄食也随之增加,由浮游性摄食方式转变为浮游−底栖性摄食。另外,因拉氏南美南极鱼摄食一定量的渔业丢弃物,导致腐生食物链和捕食食物链的贡献率特征弱化。研究结果进一步显示,针对大洋性鱼类,与肝脏和性腺相比,肌肉组织脂肪酸更适用于表征食物来源。Abstract: As one of the most abundant species of family Notothenioidei, rock cod (Patagonotothen ramsayi) plays an important role in energy transfer in the food web in the Patagonian Shelf. Currently, the work on feeding ecology of this species mainly bases on the conventional stomach content analysis, which only reflects short-term variation in feeding. Therefore, this study analyzed the distribution of fatty acids in three tissues (muscle, liver and gonad) of rock cod, and analyzed whether fatty acids in these tissues can characterize their feeding habits and transfer of diet. The results indicate that 27 kinds of fatty acids were detected in the tissues of rock cod. The storage and use of fatty acids in liver and gonad tissues are not suitable for indicating the diet of rock cod because they are involved in growth and reproduction. Compared to liver and gonad tissues, the longer renewal cycle of muscle tissue may better reflect its absorption of fatty acids from food. For the analysis of fatty acids in the muscle tissue of the rock cod in different size groups, it is inferred that the small-size rock cod (immature individual, 100−240 mm) mainly fed on planktonic organisms. The benthic feeding characteristics of rock cod increase with the increasing of size, and the feeding pattern transfer from planktonic feeding to planktonic-benthic feeding. In addition, due to the consumption of a certain amount of fishery discards by rock cod, the contribution rate of saprophytic food chain to predatory food chain is weakened. The results derive from this study further demonstrate that, compare to tissues of liver and gonad, the fatty acids of muscle tissue can be more suitable to indicate the food source of oceanic fish species.
-
Key words:
- rock cod /
- fatty acid /
- feeding habit /
- Patagonian Shelf
-
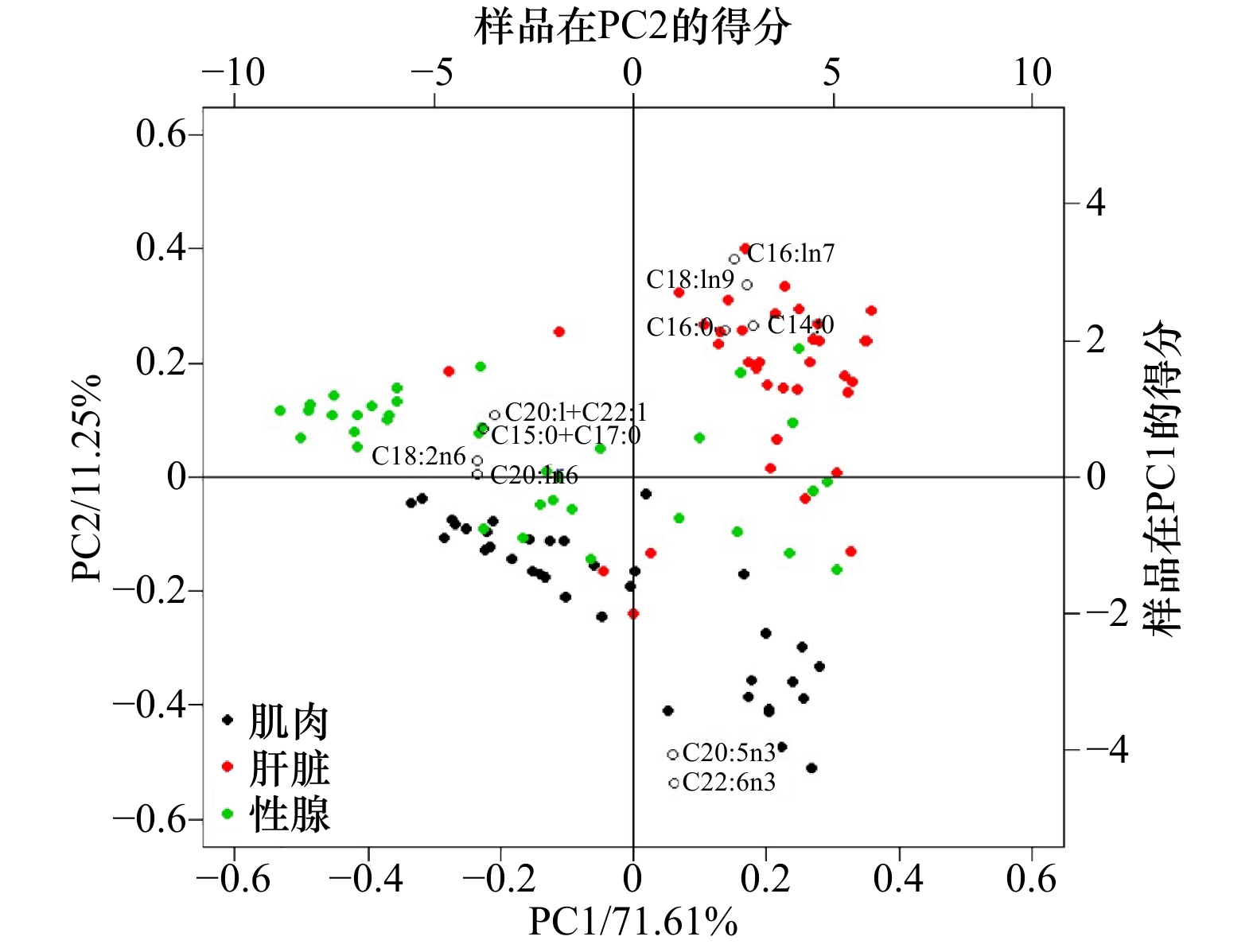
图 2 拉氏南美南极鱼不同组织脂肪酸含量主成分分析
PC1为第一主成分贡献率,PC2为第二主成分贡献率;右方纵坐标与上方横坐标分别为样品点在PC1及PC2上的得分情况
Fig. 2 Principal component analysis plot in different tissues of the rock cod
PC1 (PC2) represents the contribution ratio of the first (second) principal component; the vertical coordinate on the right and the horizontal coordinate on the top are the scores of sample points on PC1 and PC2, respectively
表 1 在拉氏南美南极鱼体内发现的主要脂肪酸及直接与间接食物源
Tab. 1 The main fatty acids found in the rock cod and their corresponding potential food sources
表 2 拉氏南美南极鱼体长分组
Tab. 2 The size group of the rock cod
体长组 体长范围 数量 小体长 100 mm≤L<240 mm 26 大体长 240 mm≤L 10 表 3 拉氏南美南极鱼不同组织中脂肪酸含量
Tab. 3 Fatty acid contents in different tissues of the rock cod
脂肪酸 平均值/mg·g−1 肌肉 肝脏 性腺 小体长 大体长 总体 小体长 大体长 总体 小体长 大体长 总体 C14:0 1.09±0.63 1.71±1.43 1.26±0.94 15.09±12.07 17.39±7.30 15.73±10.90 8.81±16.08 14.31±18.59 10.34±16.73 C15:0 0.23±0.10 0.33±0.10 0.26±0.11 1.27±0.86 0.93±0.18 1.18±0.75 1.66±1.60 1.87±2.11 1.72±1.72 C16:0 7.54±3.63 11.50±4.58 8.64±4.25 62.57±49.07 65.78±23.79 63.46±43.21 42.59±51.55 59.26±64.79 47.22±55.09 C17:0 1.37±0.80 1.64±0.69 1.45±0.77 3.14±1.03 2.67±0.19 3.01±0.90 7.94±7.37 4.07±3.10 6.86±6.66 C18:0 2.84±0.87 3.28±0.62 2.96±0.82 12.83±9.00 11.95±6.30 12.58±8.26 13.69±9.80 10.08±8.40 12.69±9.46 C20:0 1.33±0.82 1.56±0.78 1.40±0.81 2.43±0.65 2.29±0.14 2.39±0.56 7.57±7.72 3.34±2.78 6.39±6.95 C21:0 1.09±0.68 1.26±0.65 1.14±0.67 1.87±0.54 1.67±0.01 1.82±0.46 6.17±6.40 2.54±2.21 5.17±5.77 C22:0 0.47±0.29 0.55±0.27 0.49±0.29 0.82±0.23 0.74±0.01 0.80±0.20 2.21±1.94 1.15±0.98 1.91±1.78 C23:0 1.02±0.70 0.92±0.78 0.99±0.71 1.75±0.73 1.68±0.03 1.73±0.62 6.06±6.39 2.37±2.32 5.04±5.78 C24:0 0.96±0.60 1.11±0.57 1.00±0.59 1.64±0.48 1.47±0.01 1.60±0.41 5.38±5.71 2.09±2.05 4.46±5.15 SFA 17.95±6.74 23.86±5.69 19.59±6.93 103.41±70.60 106.55±36.18 104.28±62.44 102.08±83.38 101.09±100.31 101.80±86.91 C15:1n5 0.61±0.46 0.80±0.42 0.66±0.45 1.16±0.41 1.07±0.01 1.14±0.35 3.77±4.15 1.62±1.40 3.18±3.71 C16:1n7 2.40±1.24 3.32±1.41 2.65±1.34 53.82±50.09 70.35±44.18 58.41±48.48 18.61±20.97 22.26±25.20 19.62±21.91 C17:1n7 1.49±1.06 1.27±1.25 1.43±1.10 0.95±1.41 0.25±0.79 0.76±1.30 7.83±10.12 3.23±3.60 6.55±8.99 C18:1n9 5.11±3.31 7.58±4.33 5.80±3.73 133.75±146.03 151.35±86.67 138.64±131.25 38.17±49.91 54.61±66.59 42.74±54.54 C20:1 1.76±0.85 2.44±0.89 1.95±0.90 7.51±5.04 8.46±4.99 7.77±4.97 9.78±7.88 7.46±5.95 9.14±7.39 C22:1n9 1.49±0.83 2.07±0.79 1.65±0.85 3.79±1.41 4.43±1.83 3.97±1.53 8.63±7.80 7.82±8.85 8.41±7.98 C24:1n9 1.25±0.74 1.52±0.67 1.33±0.72 2.62±0.68 2.38±0.32 2.55±0.60 7.21±6.88 3.90±3.18 6.29±6.22 MUFA 14.11±5.74 19.00±6.06 15.47±6.16 203.60±197.94 238.29±130.78 213.23±180.64 94.01±81.53 100.90±107.71 95.92±87.98 C18:2n6 6.01±3.61 6.85±3.25 6.24±3.49 11.94±3.52 9.98±0.62 11.40±3.12 32.94±32.74 14.94±12.17 27.94±29.50 C18:3n6 1.49±0.92 1.73±0.87 1.56±0.90 2.64±0.73 2.36±0.06 2.56±0.63 8.43±8.64 3.60±3.11 7.09±7.78 C18:3n3 1.80±1.03 2.16±0.93 1.90±1.01 3.42±0.89 3.16±0.23 3.34±0.77 8.33±6.92 5.03±4.23 7.41±6.40 C20:2 1.46±0.85 1.71±0.77 1.53±0.82 3.04±0.77 2.74±0.16 2.96±0.67 8.14±7.97 3.80±3.05 6.94±7.19 C20:3n6 1.08±0.69 1.27±0.64 1.14±0.68 1.93±0.53 1.75±0.06 1.88±0.46 4.55±4.76 2.61±2.21 4.01±4.27 C20:3n3 3.17±1.71 3.77±1.55 3.34±1.67 6.29±2.10 5.39±0.83 6.04±1.87 16.54±16.09 7.44±5.26 14.01±14.46 C20:4n6 5.57±3.12 6.50±2.97 5.83±3.07 10.63±3.13 9.35±1.02 10.28±2.76 29.82±29.59 13.69±10.45 25.34±26.59 C20:5n3 8.86±4.86 11.24±4.76 9.52±4.89 21.04±24.76 18.50±8.59 20.33±21.41 21.35±27.64 41.97±45.10 27.08±34.00 C22:2n6 1.12±1.37 1.01±1.61 1.09±1.42 2.40±1.19 2.18±1.15 2.34±1.16 7.02±11.24 3.02±3.98 5.91±9.88 C22:6n3 26.48±12.84 32.61±12.38 28.18±12.84 46.06±64.40 43.11±24.99 45.24±55.90 36.19±47.71 82.41±85.98 49.03±62.99 PUFA 57.05±18.25 68.85±13.76 60.33±17.76 109.40±92.23 98.51±34.06 106.37±79.99 173.30±138.01 178.51±157.40 174.75±141.35 C15:0+C17:0 1.60±0.88 1.97±0.71 1.71±0.84 4.42±1.77 3.59±0.36 4.19±1.55 9.60±8.05 5.94±4.81 8.58±7.42 C20:1+C22:1 3.26±1.67 4.51±1.65 3.61±1.74 11.30±6.30 12.89±6.79 11.74±6.38 18.41±15.56 15.28±14.42 17.54±15.11 C20:5n3 / C22:6n3 0.35±0.16 0.36±0.13 0.35±0.15 0.59±0.20 0.47±0.11 0.55±0.19 0.62±0.20 0.46±0.15 0.58±0.20 n-3 PUFA 40.31±16.37 49.78±14.49 42.95±16.25 76.80±89.37 70.16±33.66 74.95±77.50 82.41±79.43 136.86±135.07 97.53±99.04 n-6 PUFA 15.28±9.02 17.36±6.83 15.85±8.42 29.55±7.37 25.62±2.07 28.46±6.57 82.75±83.90 37.85±31.49 70.28±75.49 TFA 89.12±28.89 111.70±23.94 95.39±29.14 416.40±322.22 443.36±178.00 423.89±287.16 369.39±285.84 380.50±359.57 372.48±302.71 注:SFA为饱和脂肪酸,MUFA为单不饱和脂肪酸,PUFA为多不饱和脂肪酸,TFA为总脂肪酸;±后为标准偏差。 表 4 拉氏南美南极鱼体长与特征脂肪酸含量之间的关系
Tab. 4 The relationship between standard length and percentage of specific fatty acids in the different tissues of rock cod
脂肪酸 肌肉 肝脏 性腺 R2 p R2 p R2 p C14:0 0.020 0.412 0.000 0.976 0.000 0.915 C16:0 0.129 0.031* 0.009 0.575 0.000 0.917 C15:0+C17:0 0.125 0.035* 0.138 0.026* 0.230 0.003* C16:1n7 0.032 0.295 0.010 0.554 0.001 0.848 C18:1n9 0.021 0.395 0.004 0.720 0.002 0.797 C18:2n6 0.095 0.067 0.235 0.003* 0.251 0.002* C20:1+C22:1 0.232 0.003* 0.019 0.417 0.152 0.019* C20:4n6 0.097 0.064 0.225 0.003* 0.240 0.002* C20:5n3 0.016 0.462 0.001 0.856 0.025 0.362 C22:6n3 0.011 0.551 0.002 0.795 0.061 0.148 C20:5n3/ C20:5n3 0.010 0.553 0.089 0.078 0.326 0.000* n-6 PUFA/n-3 PUFA 0.004 0.706 0.034 0.280 0.252 0.002* 注:*表示两者关系呈线性关系(p<0.05)。 -
[1] Parkes G. Fishes of the Southern Ocean[J]. Reviews in Fish Biology and Fisheries, 1992, 2(4): 344−345. doi: 10.1007/BF00043525 [2] Ekau W. Biological investigations on Notothenia ramsayi Regan 1913 (Pisces, Notothenioidei, Nototheniidae)[J]. Archiv für Fischereiwissenschaft, 1982, 33(1/2): 43−68. [3] Sosiński J, Janusz J. The distribution and biology of Patagonotothen ramsayi (Regan, 1913): results of Polish studies on the Patagonian Shelf, 1979-1993[R]. Gdynia, Poland: Sea Fisheries Research Institute, 2003. [4] Brickle P, Arkhipkin A, Shcherbich Z. Age and growth of a sub-Antarctic notothenioid, Patagonotothen ramsayi (Regan 1913), from the Falkland Islands[J]. Polar Biology, 2006, 29(8): 633−639. doi: 10.1007/s00300-005-0099-9 [5] Hart T J. Report on trawling surveys on the Patagonian continental shelf[J]. Discovery Reports, 1946, 23: 223−408. [6] Laptikhovsky V V, Arkhipkin A I. An impact of seasonal squid migrations and fishing on the feeding spectra of subantarctic notothenioids Patagonotothen ramsayi and Cottoperca gobio around the Falkland Islands[J]. Journal of Applied Ichthyology, 2003, 19(1): 35−39. doi: 10.1046/j.1439-0426.2003.00340.x [7] Laptikhovsky V V. A comparative study of diet in three sympatric populations of Patagonotothen species (Pisces: Nototheniidae)[J]. Polar Biology, 2004, 27(4): 202−205. doi: 10.1007/s00300-003-0573-1 [8] Padovani L N, Viñas M D, Sánchez F, et al. Amphipod-supported food web: Themisto gaudichaudii, a key food resource for fishes in the southern Patagonian Shelf[J]. Journal of Sea Research, 2012, 67(1): 85−90. doi: 10.1016/j.seares.2011.10.007 [9] Arkhipkin A, Laptikhovsky V. From gelatinous to muscle food chain: rock cod Patagonotothen ramsayi recycles coelenterate and tunicate resources on the Patagonian Shelf[J]. Journal of Fish Biology, 2013, 83(5): 1210−1220. doi: 10.1111/jfb.12217 [10] Clausen A, Pütz K. Winter diet and foraging range of gentoo penguins (Pygoscelis papua) from Kidney Cove, Falkland Islands[J]. Polar Biology, 2003, 26(1): 32−40. doi: 10.1007/s00300-002-0443-2 [11] La Mesa M, Riginella E, Melli V, et al. Biological traits of a sub-Antarctic nototheniid, Patagonotothen ramsayi, from the Burdwood Bank[J]. Polar Biology, 2016, 39(1): 103−111. doi: 10.1007/s00300-015-1663-6 [12] Hobson K A, Piatt J F, Pitocchelli J. Using stable isotopes to determine seabird trophic relationships[J]. Journal of Animal Ecology, 1994, 63(4): 786−798. doi: 10.2307/5256 [13] Cherel Y, Hobson K A, Weimerskirch H. Using stable isotopes to study resource acquisition and allocation in procellariiform seabirds[J]. Oecologia, 2005, 145(4): 533−540. doi: 10.1007/s00442-005-0156-7 [14] Connan M, Mayzaud P, Boutoute M, et al. Lipid composition of stomach oil in a procellariiform seabird Puffinus tenuirostris: implications for food web studies[J]. Marine Ecology Progress Series, 2005, 290: 277−290. doi: 10.3354/meps290277 [15] Tierney M, Nichols P D, Wheatley K E, et al. Blood fatty acids indicate inter- and intra-annual variation in the diet of Adélie penguins: comparison with stomach content and stable isotope analysis[J]. Journal of Experimental Marine Biology and Ecology, 2008, 367(2): 65−74. doi: 10.1016/j.jembe.2008.07.046 [16] Cowey C B, Sargent J R. Fish nutrition[J]. Advances in Marine Biology, 1972, 10: 383−494. doi: 10.1016/S0065-2881(08)60419-8 [17] dos Santos J, Burkow I C, Jobling M. Patterns of growth and lipid deposition in cod (Gadus morhua L.) fed natural prey and fish-based feeds[J]. Aquaculture, 1993, 110(2): 173−189. doi: 10.1016/0044-8486(93)90271-Y [18] Beckmann C L, Mitchell J G, Seuront L, et al. Experimental evaluation of fatty acid profiles as a technique to determine dietary composition in benthic elasmobranchs[J]. Physiological and Biochemical Zoology, 2013, 86(2): 266−278. doi: 10.1086/669539 [19] González M J, Gallardo J M, Brickle P, et al. Nutritional composition and safety of Patagonotothen ramsayi, a discard species from Patagonian Shelf[J]. International Journal of Food Science and Technology, 2007, 42(10): 1240−1248. doi: 10.1111/j.1365-2621.2006.01472.x [20] Folch J, Lees M, Sloane Stanley G H. A simple method for the isolation and purification of total lipides from animal tissues[J]. Journal of Biological Chemistry, 1957, 226(1): 497−509. [21] Parrish C C, Abrajano T A, Budge S M, et al. Lipid and phenolic biomarkers in marine ecosystems: analysis and applications[M]//Wangersky P J. Marine Chemistry. Berlin, Heidelberg: Springer, 2000. [22] Rajendran N, Suwa Y, Urushigawa Y. Distribution of phospholipid ester-linked fatty acid biomarkers for bacteria in the sediment of Ise Bay, Japan[J]. Marine Chemistry, 1993, 42(1): 39−56. doi: 10.1016/0304-4203(93)90248-M [23] Pond D W, Bell M V, Harris R P, et al. Microplanktonic polyunsaturated fatty acid markers: a mesocosm trial[J]. Estuarine, Coastal and Shelf Science, 1998, 46(2): 61−67. doi: 10.1006/ecss.1998.0334 [24] Johns R B, Nichols P D, Perry G J. Fatty acid composition of ten marine algae from Australian waters[J]. Phytochemistry, 1979, 18(5): 799−802. doi: 10.1016/0031-9422(79)80018-7 [25] Napolitano G E, Pollero R J, Gayoso A M, et al. Fatty acids as trophic markers of phytoplankton blooms in the Bahía Blanca estuary (Buenos Aires, Argentina) and in Trinity Bay (Newfoundland, Canada)[J]. Biochemical Systematics and Ecology, 1997, 25(8): 739−755. doi: 10.1016/S0305-1978(97)00053-7 [26] Falk-Petersen S, Sargent J R, Tande K S. Lipid composition of zooplankton in relation to the sub-Arctic food web[J]. Polar Biology, 1987, 8(2): 115−120. doi: 10.1007/BF00297065 [27] Nogueira N, Fernandes I, Fernandes T, et al. A comparative analysis of lipid content and fatty acid composition in muscle, liver and gonads of Seriola fasciata Bloch 1793 based on gender and maturation stage[J]. Journal of Food Composition and Analysis, 2017, 59: 68−73. doi: 10.1016/j.jfca.2016.11.005 [28] Dalsgaard A J T. Fatty acid trophic markers as measures of energy transfer through marine food webs exemplified by sandeel[D]. Copenhagen: University of Copenhagen, 2003. [29] Grubert M A, Dunstan G A, Ritar A J. Lipid and fatty acid composition of pre- and post-spawning blacklip (Haliotis rubra) and greenlip (Haliotis laevigata) abalone conditioned at two temperatures on a formulated feed[J]. Aquaculture, 2004, 242(1/4): 297−311. [30] 施瑔芳. 鱼类性腺发育研究新进展[J]. 水生生物学报, 1988, 12(3): 248−258.Shi Quanfang. Recent advances in the studies on gonad development in fishes[J]. Acta Hydrobiologica Sinica, 1988, 12(3): 248−258. [31] Watanabe T, Kitajima C, Fujita S. Nutritional values of live organisms used in Japan for mass propagation of fish: a review[J]. Aquaculture, 1983, 34(1/2): 115−143. [32] 朱国平, 许柳雄, 陈新军. 西南大西洋拉氏南美南极鱼生物学特性的初步研究[J]. 水产学报, 2010, 34(12): 1877−1882.Zhu Guoping, Xu Liuxiong, Chen Xinjun. The biology characteristics of rockcod (Patagonotothen ramsayi) in the Southwestern Atlantic Ocean[J]. Journal of Fisheries of China, 2010, 34(12): 1877−1882. [33] Pond D, Harris R, Head R, et al. Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK[J]. Marine Ecology Progress Series, 1996, 143(1/3): 45−63. [34] Bell M V, Batty R S, Dick J R, et al. Dietary deficiency of docosahexaenoic acid impairs vision at low light intensities in juvenile herring (Clupea harengus L.)[J]. Lipids, 1995, 30(5): 443−449. doi: 10.1007/BF02536303 [35] Estévez A, McEvoy L A, Bell J G, et al. Growth, survival, lipid composition and pigmentation of turbot (Scophthalmus maximus) larvae fed live-prey enriched in Arachidonic and Eicosapentaenoic acids[J]. Aquaculture, 1999, 180(3/4): 321−343. [36] Jeffries H P. Seasonal composition of temperate plankton communities: fatty acids[J]. Limnology and Oceanography, 1970, 15(3): 419−426. doi: 10.4319/lo.1970.15.3.0419 [37] Rossi S, Youngbluth M J, Jacoby C A, et al. Fatty acid trophic markers and trophic links among seston, crustacean zooplankton and the siphonophore Nanomia cara in Georges Basin and Oceanographer Canyon (NW Atlantic)[J]. Scientia Marina, 2008, 72(2): 403−416. [38] Budge S M, Parrish C C. Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland. II. Fatty acids[J]. Organic Geochemistry, 1998, 29(5/7): 1547−1559. [39] Zhu Guoping, Zhang Haiting, Yang Yang, et al. Upper trophic structure in the Atlantic Patagonian shelf break as inferred from stable isotope analysis[J]. Journal of Oceanology and Limnology, 2018, 36(3): 717−725. doi: 10.1007/s00343-018-6340-5 [40] Laptikhovsky V, Arkhipkin A, Brickle P. From small bycatch to main commercial species: explosion of stocks of rock cod Patagonotothen ramsayi (Regan) in the Southwest Atlantic[J]. Fisheries Research, 2013, 147: 399−403. doi: 10.1016/j.fishres.2013.05.006 [41] Kock K H. Antarctic Fish and Fisheries[M]. Cambridge: Cambridge University Press, 1992. [42] Fukuda Y, Naganuma T. Potential dietary effects on the fatty acid composition of the common jellyfish Aurelia aurita[J]. Marine Biology, 2001, 138(5): 1029−1035. doi: 10.1007/s002270000512 -




 下载:
下载: