Sequential extraction of Sr and Nd isotope from Fe–Mn oxyhydroxide and detrital in marine sediments
-
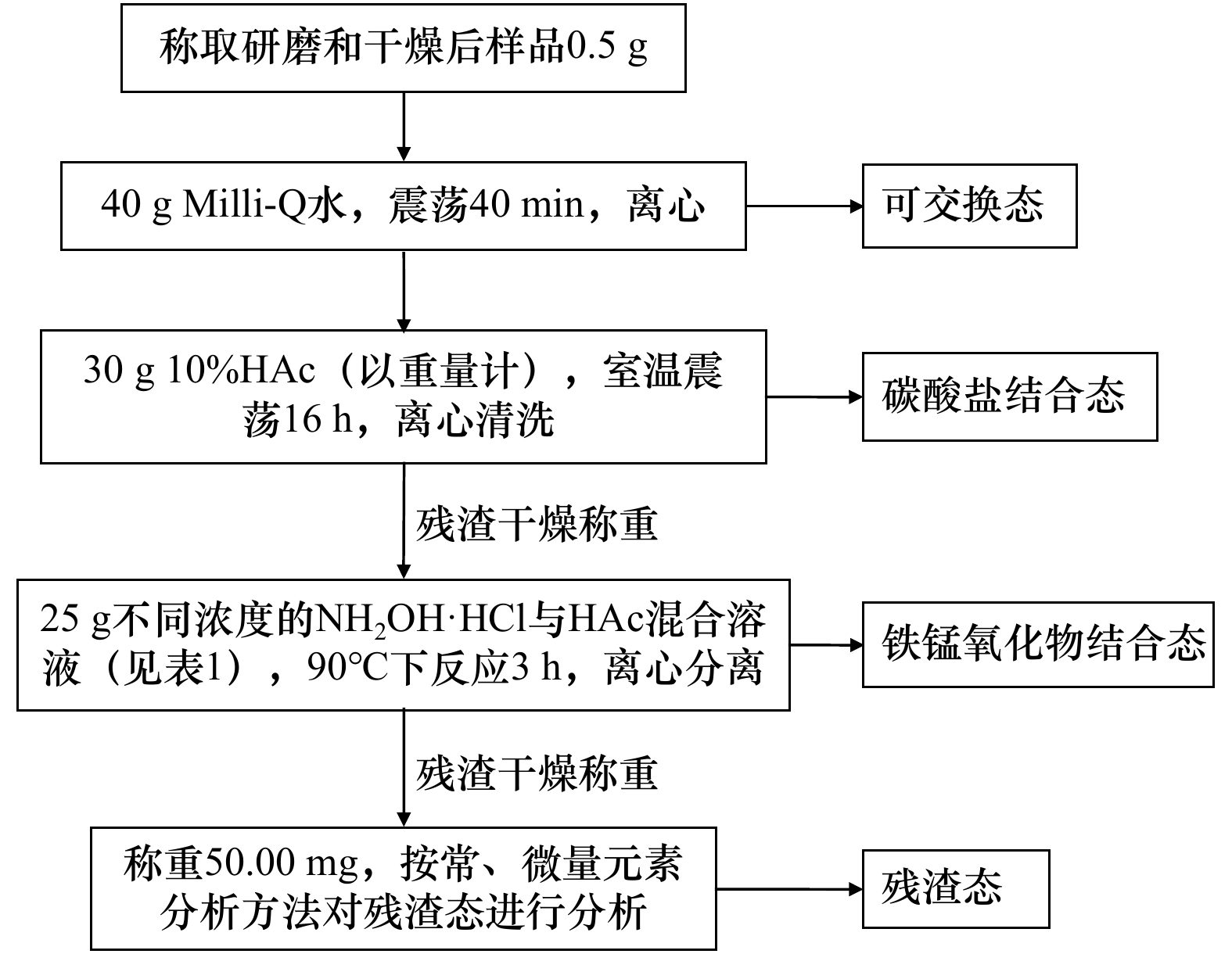
摘要: 海洋沉积物中Fe-Mn氧化物相和残渣态的Sr、Nd同位素组成能够敏感地指示洋流循环及物质来源,但实验室对沉积物中上述相态的Sr和Nd同位素的提取易产生过量或提取不完全,进而影响同位素测定结果的准确性,因此制定有效的提取流程显得非常重要。本文采用不同浓度盐酸羟胺(Hydroxylamine Hydrochloride,HH)与醋酸(Acetic Acid,HAc)混合溶液对中印度洋海盆深海沸石黏土、北极半深海沉积物以及安达曼海近海沉积物的Fe-Mn氧化物相进行提取,残渣态用HNO3-HF高压密闭消解法溶融,测定了各相态的主微量元素含量及Sr、Nd同位素组成,通过分析不同实验条件下得到的Fe-Mn氧化物相与残渣态的稀土元素(REE)配分模式、Al/Nd含量比值及Sr、Nd同位素组成,建立了3种不同成因类型海洋沉积物不同相态的化学提取方法。提取深海沸石黏土中Fe-Mn氧化物相的理想试剂条件为0.25 mol/L HH和15% HAc,北极半深海沉积物和安达曼海近海沉积物的试剂条件为0.5 mol/L HH和15% HAc。该方法可以准确获得沉积物中Fe-Mn氧化物相与残渣态的Sr、Nd同位素组成信息,为古海洋学的研究提供方法支持。Abstract: The radiogenic isotope composition of neodymium (Nd) and strontium (Sr) extracted from Fe-Mn oxyhydroxide and detrital in marine sediments indicated potential for investigate present and past oceanic circulation or input of terrigenous material. However, the isotope compositions of elements obtained from the Fe-Mn oxyhydroxide fraction and detrital are easily disturbed by each other originating from the extraction process, will affect the isotope composition of these fractions. Therefore, it is very important to establish a rigorous leaching procedure that can be used to separate both Fe-Mn oxyhydroxide and the detrital fraction from the same marine sediment sample for Nd and Sr isotopic analysis. In this study, the mixture reagent of hydroxylamine hydrochloride (HH) and acetic acid (HAc) at 12 different concentrations were used to extract Fe-Mn oxyhydroxide fraction and detrital from zeolite clay of the Central Indian Ocean Basin, bathyal sediment of Arctic and offshore marine sediment of the Andaman Sea. Detrital was dissolved by HF-HNO3 system with high-pressure closed digestion method. Elements concentration and Sr and Nd isotope ratios in these fractions were measured. To corroborate the reliability of the extracting methods, REE patterns, Al/Ca ratios, as well as Sr and Nd isotope compositions were applied to assess the absence of detrital contributions to the extracted solutions and to support the seawater origin of the Nd isotope ratios in the Fe-Mn oxyhydroxide fraction. The result showed that different genetic types of sediments have different extraction reagents. The ideal reagent concentration for extraction of Fe-Mn oxyhydroxide fraction from zeolite clay is 0.25 mol/L HH in 15% acetic acid, for bathyal sediment of Greenland Sea and offshore marine sediment is 0.5 mol/L HH in 15% acetic acid. This method can accurately obtain the Sr and Nd isotopic composition of Fe-Mn oxyhydroxide and residue state in marine sediments, providing method support for the study of paleoceanography.
-
Key words:
- marine sediment /
- extraction /
- Fe-Mn oxyhydroxide /
- detritus /
- 87Sr/86Sr /
- 143Nd/144Nd
-
表 1 3个沉积物样品采样信息和特征
Tab. 1 Sample locations and characteristics of sediments analyzed in this study
实验编号 样品号 海区 纬度 经度 水深/m 粒级组分/% 沉积物类型 砂 粉砂 黏土 A GC19 中印度洋海盆 3°53′S 8°55′E 4 643 0.03 68.84 31.16 深海黏土(含有铁锰微结核) B IS4C 格陵兰海 68°42′N 14°48’W 1 595 5.36 61.98 32.66 黏土质粉砂 C ADM-S18 安达曼海 6°57’S 97°55′E 335 20.05 71.09 8.88 砂质粉砂 表 2 3个样品中主、微量元素及稀土元素含量(主量元素单位:%, 微量元素单位:10-6)
Tab. 2 Contents of major(%), trace and rare earth elements(10-6) of 3 samples from different areas
样品 Al2O3 CaO Fe2O3 K2O MgO MnO Na2O P2O5 TiO2 SiO2 Ba Sr Zn Zr Cr Co Ni A 11.38 4.14 13.22 2.68 3.12 3.76 5.02 2.59 0.59 38.32 404 322 243 200 43 249 765 B 10.34 16.52 5.99 1.87 2.28 0.21 3.55 0.21 0.86 38.37 593 654 77 132 60 23 35 C 10.86 7.49 3.63 1.85 2.05 0.04 3.22 0.13 0.49 50.71 217 317 75 190 54 9 27 样品 Cu Y La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ΣREE A 680 434 271 212 65.6 281 60.72 14.50 56.8 10.61 66.9 13.04 37.25 5.51 33.67 5.53 1 133 B 30 23 28 57 6.7 25 4.95 1.35 4.46 0.74 4.57 0.85 2.38 0.38 2.33 0.35 139 C 18 24 31 63 7.1 26 5.04 0.91 4.30 0.73 4.40 0.83 2.44 0.39 2.47 0.39 149 表 3 不同浓度盐酸羟氨(CH·H)与不同质量百分比浓度醋酸(CHAc)混合溶液条件
Tab. 3 The mixture of hydroxylamine hydrochloride and acetic acid at different concentrations
编号 CH·H/mol·L−1 CHAc/% 编号 CH·H /mol·L−1 CHAc/% a1 2.00 15 b1 2.00 25 a2 1.00 15 b2 1.00 25 a3 0.50 15 b3 0.50 25 a4 0.25 15 b4 0.25 25 a5 0.10 15 b5 0.10 25 a6 0.04 15 b6 0.04 25 表 4 ICP-OES标准溶液浓度
Tab. 4 Concentrations of standard solutions measured by ICP-OES
溶液 混合标液编号 空白 STD 1 STD 2 STD 3 STD 4 多元素混合标准溶液1/μg·mL−1 0.000 0.025 0.125 0.250 1.250 多元素混合标准溶液 2/μg·mL−1 0.000 0.100 0.500 1.000 5.000 多元素混合标准溶液4/μg·mL−1 0.000 0.025 0.125 0.250 1.250 表 5 ICP-MS标准溶液浓度
Tab. 5 Concentrations of standard solutions measured by ICP-MS
溶液 混合标液编号 空白 STD 1 STD 2 STD 3 STD 4 STD 5 多元素混合标准溶液 1/ng·mL−1 0.00 0.50 2.50 5.00 25.00 50.00 多元素混合标准溶液 2/ng·mL−1 0.00 1.00 5.00 10.00 50.00 100.00 多元素混合标准溶液 4/ng·mL−1 0.00 1.00 5.00 10.00 50.00 100.00 表 6 不同提取条件下Fe-Mn氧化物相和残渣态元素在全样中的百分含量(%)
Tab. 6 Percentage of representative elements in different chemical phase from leaching tests(%)
样品 Al Ca Fe Mn Sr Nd Fe-Mn
氧化物相残渣态 Fe-Mn
氧化物相残渣态 Fe-Mn
氧化物相残渣态 Fe-Mn
氧化物相残渣态 Fe-Mn
氧化物相残渣态 Fe-Mn
氧化物相残渣态 Aa1 3.85 74.41 20.97 6.41 18.90 64.47 69.27 1.63 24.21 13.39 89.25 8.47 Aa2 2.94 79.29 24.00 11.30 16.58 70.33 70.85 2.32 29.31 15.40 78.94 22.18 Aa3 2.57 79.77 22.98 15.31 15.20 70.27 71.23 2.97 29.66 17.18 68.01 32.84 Aa4 2.35 82.31 20.80 16.27 15.44 73.57 74.84 3.77 29.64 18.30 55.01 39.24 Aa5 2.02 80.89 17.34 19.02 13.42 74.22 72.81 4.46 26.41 20.05 41.92 47.78 Aa6 2.12 77.81 13.15 16.17 9.75 77.66 68.83 5.68 20.08 19.55 39.32 43.71 Ab1 4.16 78.86 28.71 7.82 20.03 65.61 70.54 1.46 31.83 14.17 87.82 13.71 Ab2 3.51 79.27 29.08 10.59 17.72 68.76 73.57 2.02 34.78 15.26 83.04 20.81 Ab3 3.04 78.96 23.05 15.16 15.60 71.30 71.89 2.63 30.16 16.87 63.51 32.52 Ab4 2.90 78.48 18.52 14.73 14.89 68.84 72.13 2.86 26.81 17.34 56.14 32.85 Ab5 2.61 77.94 18.22 23.32 12.98 71.60 70.41 3.53 27.50 20.75 41.00 54.47 Ab6 2.56 78.16 13.89 24.33 10.29 78.28 67.42 5.12 21.92 22.25 35.13 55.45 Ba1 2.79 73.04 1.39 7.15 13.52 72.17 57.92 17.40 2.57 15.25 21.03 60.89 Ba2 3.02 74.83 1.51 8.39 13.52 74.87 60.79 20.02 2.78 16.24 20.63 62.41 Ba3 2.82 76.16 1.49 7.18 11.84 75.14 60.94 20.97 2.75 16.00 17.30 62.84 Ba4 2.75 74.39 1.54 9.27 11.17 74.63 61.72 21.79 2.74 17.38 15.87 68.06 Ba5 2.41 69.88 1.81 9.07 9.34 70.64 61.47 21.37 2.66 16.89 12.58 69.46 Ba6 2.13 75.66 1.56 9.90 7.68 77.46 60.13 23.73 2.50 18.38 10.42 74.77 Bb1 3.15 74.53 1.52 9.27 15.02 70.18 61.09 20.08 2.69 17.72 22.40 61.46 Bb2 2.96 72.56 1.41 9.60 13.05 70.72 59.34 20.70 2.60 17.52 19.92 65.92 Bb3 3.19 71.04 1.53 8.94 13.18 71.29 60.97 20.10 2.71 16.88 18.42 66.93 Bb4 2.49 74.03 1.23 9.68 9.81 74.43 52.69 21.77 2.22 17.78 12.97 64.60 Bb5 2.40 73.56 1.31 9.75 9.38 73.72 57.13 21.94 2.25 18.19 12.19 72.92 Bb6 2.32 70.02 1.37 9.24 8.57 74.95 57.32 21.81 2.30 16.73 10.57 70.51 Ca1 2.76 76.38 3.50 1.48 20.46 63.62 19.54 25.69 3.70 9.26 25.14 54.90 Ca2 2.42 76.86 3.38 1.44 18.36 65.49 18.50 26.84 3.56 9.35 20.27 60.77 Ca3 1.98 78.41 3.30 1.46 16.31 68.99 16.74 27.80 3.44 9.51 16.99 65.14 Ca4 1.50 88.44 3.45 1.27 14.22 84.72 15.98 31.54 3.58 9.27 11.81 73.32 Ca5 1.11 84.03 3.18 1.47 12.66 80.19 14.61 30.73 3.34 9.29 8.23 72.85 Ca6 0.85 77.62 3.13 1.81 10.33 77.92 26.23 30.76 3.16 9.46 5.37 75.11 Cb1 3.07 79.39 3.27 1.28 22.11 65.14 19.26 24.99 3.37 8.99 23.97 56.20 Cb2 2.54 80.61 2.95 1.54 18.05 68.60 18.62 26.52 3.16 9.28 20.08 59.01 Cb3 1.94 74.29 2.71 1.50 14.67 65.01 15.75 26.52 2.86 9.23 14.27 59.30 Cb4 2.28 97.25 3.10 0.90 19.03 94.02 18.13 30.04 3.28 9.01 15.04 70.71 Cb5 1.29 74.53 3.12 1.71 13.34 67.18 15.25 28.49 3.17 9.34 8.69 65.65 Cb6 0.89 68.32 3.04 1.97 10.58 63.13 13.41 27.92 3.06 9.53 5.89 68.85 表 7 不同提取条件下Fe-Mn氧化物相和残渣态中元素浓度及Sr、Nd同位素比值
Tab. 7 Al, Sr and Nd concentration and Sr, Nd isotope ratios of Fe-Mn oxyhydroxide leaching phases and the respective detrital fraction
样品 Fe-Mn 氧化物相 样品 残渣态 Al/% Sr/10−6 Nd/10−6 87Sr/86Sr 143Nd/144Nd εNd Al/Nd Al/% Sr/10−6 Nd/10−6 87Sr/86Sr 143Nd/144Nd εNd Al/Nd ±0.000 02 ±0.000 014 ±0.27 ±0.000 02 ±0.000 014 ±0.27 Aa1 0.29 97 290 0.709 63 0.512 285 −6.9 9.9 Aa1 6.59 63 39 0.719 05 0.512 207 −8.4 1 671 Aa2 0.21 113 239 0.710 11 0.512 278 −7.0 8.8 Aa2 6.69 69 98 0.717 95 0.512 249 −7.6 680 Aa3 0.18 114 205 0.70925 0.512 279 −7.0 9.0 Aa3 6.63 76 143 0.716 95 0.512 265 −7.3 462 Aa4 0.17 116 181 0.70925 0.512 279 −7.0 9.5 Aa4 6.72 80 168 0.717 28 0.512 267 −7.2 399 Aa5 0.15 102 136 0.70979 0.512 282 −6.9 10.7 Aa5 6.58 87 204 0.716 10 0.512 264 −7.3 322 Aa6 0.16 80 134 0.709 91 0.512 274 −7.1 11.9 Aa6 6.49 87 192 0.716 37 0.512 267 −7.2 339 Ab1 0.30 121 286 0.710 70 0.512 275 −7.1 10.4 Ab1 6.69 64 61 0.718 85 0.512 230 −8.0 1 094 Ab2 0.25 133 265 0.709 38 0.512 274 −7.1 9.5 Ab2 6.68 69 92 0.718 14 0.512 254 −7.5 725 Ab3 0.22 115 204 0.709 24 0.512 284 −6.9 10.6 Ab3 6.55 75 142 0.717 25 0.512 270 −7.2 462 Ab4 0.21 106 185 0.709 72 0.512 272 −7.1 11.5 Ab4 6.58 78 145 0.716 97 0.512 258 −7.4 455 Ab5 0.19 109 134 0.70961 0.512 271 −7.2 14.4 Ab5 6.53 93 240 0.715 63 0.512 267 −7.2 272 Ab6 0.18 85 112 0.709 21 0.512 277 −7.0 16.5 Ab6 6.38 97 238 0.716 12 0.512 271 −7.2 268 Ba1 0.25 27 8.32 0.709 04 0.512 443 −3.8 296 Ba1 6.56 164 29 0.711 93 0.512 232 −7.9 2 294 Ba2 0.26 28 7.99 0.708 63 0.512 457 −3.5 324 Ba2 6.67 173 29 0.711 46 0.512 311 −6.4 2 293 Ba3 0.24 29 6.72 0.708 68 0.512 461 −3.5 363 Ba3 6.83 172 29 0.711 71 0.512 277 −7.1 2 318 Ba4 0.24 28 6.18 0.70858 0.512 449 −3.7 387 Ba4 6.62 185 32 0.710 99 0.512 357 −5.5 2 090 Ba5 0.21 27 4.80 0.70854 0.512 443 −3.8 429 Ba5 6.20 179 32 0.710 72 0.512 366 −5.3 1 924 Ba6 0.18 26 4.07 0.708 53 0.512 436 −3.9 450 Ba6 6.62 192 34 0.710 71 0.512 356 −5.5 1 935 Bb1 0.27 28 8.67 0.708 70 0.512 453 −3.6 313 Bb1 6.69 190 29 0.710 90 0.512 320 −6.2 2 319 Bb2 0.25 27 7.59 0.708 70 0.512 456 −3.6 335 Bb2 6.52 188 31 0.710 80 0.512 326 −6.1 2 105 Bb3 0.28 28 7.30 0.708 62 0.512 447 −3.7 381 Bb3 6.38 181 31 0.711 01 0.512 344 −5.7 2 030 Bb4 0.22 23 5.09 0.708 59 0.512447 −3.7 423 Bb4 6.57 189 30 0.710 82 0.512 355 −5.5 2 191 Bb5 0.21 23 4.65 0.708 57 0.512 444 −3.8 447 Bb5 6.52 193 34 0.710 68 0.512 327 −6.1 1 929 Bb6 0.20 24 4.15 0.708 52 0.512 444 −3.8 491 Bb6 6.21 177 33 0.710 94 0.512 340 −5.8 1 899 Ca1 0.20 15 8.21 0.709 62 0.512 127 −10.0 249 Ca1 5.93 40 22 0.727 62 0.511 989 −12.7 2 723 Ca2 0.18 15 6.73 0.709 62 0.512 131 −9.9 266 Ca2 5.96 40 24 0.727 64 0.511 977 −12.9 2 476 Ca3 0.14 14 5.46 0.709 55 0.512 136 −9.8 264 Ca3 6.01 40 26 0.727 42 0.511 992 −12.6 2 356 Ca4 0.11 15 3.73 0.709 37 0.512 142 −9.7 296 Ca4 6.78 39 29 0.726 80 0.512 017 −12.1 2 362 Ca5 0.08 14 2.64 0.709 39 0.512 149 −9.5 311 Ca5 6.46 39 29 0.727 45 0.512 025 −12.0 2 258 Ca6 0.06 13 1.73 0.709 33 0.512 137 −9.8 358 Ca6 5.94 40 29 0.726 41 0.512 001 −12.4 2 023 Cb1 0.23 14 7.77 0.709 39 0.512 144 −9.6 290 Cb1 6.14 38 22 0.728 08 0.511 960 −13.2 2 765 Cb2 0.19 13 6.50 0.709 67 0.512 124 −10.0 285 Cb2 6.18 39 23 0.727 32 0.511 991 −12.6 2 674 Cb3 0.14 12 4.60 0.709 46 0.512 136 −9.8 308 Cb3 5.64 39 23 0.727 56 0.511 967 −13.1 2 453 Cb4 0.17 13 4.84 0.709 44 0.512 141 −9.7 347 Cb4 7.50 38 28 0.726 75 0.512 021 −12.0 2 692 Cb5 0.10 13 2.86 0.709 44 0.512 136 −9.8 339 Cb5 5.79 40 26 0.727 38 0.512 026 −11.9 2 223 Cb6 0.07 12 1.92 0.709 36 0.512 131 −9.9 340 Cb6 5.16 40 27 0.726 55 0.512 005 −12.4 1 942 -
[1] Franzese A M, Hemming S R, Goldstein S L, et al. Reduced Agulhas leakage during the last glacial maximum inferred from an integrated provenance and flux study[J]. Earth and Planetary Science Letters, 2006, 250(1/2): 72−88. [2] Hemming S R, van de Flierdt T, Goldstein S L, et al. Strontium isotope tracing of terrigenous sediment dispersal in the Antarctic Circumpolar Current: implications for constraining frontal positions[J]. Geochemistry, Geophysics, Geosystems, 2007, 8(6): Q06N13. [3] Stumpf R, Frank M, Schönfeld J, et al. Climatically driven changes in sediment supply on the SW Iberian shelf since the Last Glacial Maximum[J]. Earth and Planetary Science Letters, 2011, 312(1/2): 80−90. [4] Delmonte B, Basile-Doelsch I, Petit J R, et al. Comparing the Epica and Vostok dust records during the last 220, 000 years: stratigraphical correlation and provenance in glacial periods[J]. Earth-Science Reviews, 2004, 66(1/2): 63−87. [5] Stichel T, Frank M, Rickli J, et al. The hafnium and neodymium isotope composition of seawater in the Atlantic sector of the Southern Ocean[J]. Earth and Planetary Science Letters, 2012, 317-318: 282−294. doi: 10.1016/j.jpgl.2011.11.025 [6] Molina-Kescher M, Frank M, Hathorne E. South Pacific dissolved Nd isotope compositions and rare earth element distributions: water mass mixing versus biogeochemical cycling[J]. Geochimica et Cosmochimica Acta, 2014, 127: 171−189. doi: 10.1016/j.gca.2013.11.038 [7] Pahnke K, Goldstein S L, Hemming S R. Abrupt changes in Antarctic intermediate water circulation over the past 25, 000 years[J]. Nature Geoscience, 2008, 1(12): 870−874. doi: 10.1038/ngeo360 [8] Rutberg R L, Hemming S R, Goldstein S L. Reduced north Atlantic deep water flux to the glacial southern ocean inferred from neodymium isotope ratios[J]. Nature, 2000, 405(6789): 935−938. doi: 10.1038/35016049 [9] Bayon G, German C R, Boella R M, et al. An improved method for extracting marine sediment fractions and its application to Sr and Nd isotopic analysis[J]. Chemical Geology, 2002, 187(3/4): 179−199. [10] Gutjahr M, Frank M, Stirling C H, et al. Reliable extraction of a deepwater trace metal isotope signal from Fe–Mn oxyhydroxide coatings of marine sediments[J]. Chemical Geology, 2007, 242(3/4): 351−370. [11] Palmer M R, Elderfield H. Rare earth elements and neodymium isotopes in ferromanganese oxide coatings of Cenozoic foraminifera from the Atlantic Ocean[J]. Geochimica et Cosmochimica Acta, 1986, 50(3): 409−417. doi: 10.1016/0016-7037(86)90194-8 [12] Asahara Y, Tanaka T, Kamioka H, et al. Provenance of the north Pacific sediments and process of source material transport as derived from Rb–Sr isotopic systematics[J]. Chemical Geology, 1999, 158(3/4): 271−291. [13] Asahara Y, Takeuchi F, Nagashima K, et al. Provenance of terrigenous detritus of the surface sediments in the Bering and Chukchi seas as derived from Sr and Nd isotopes: implications for recent climate change in the Arctic regions[J]. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 2012, 61-64: 155−171. doi: 10.1016/j.dsr2.2011.12.004 [14] Grousset F E, Parra M, Bory A, et al. Saharan wind regimes traced by the Sr–Nd isotopic composition of subtropical Atlantic sediments: last glacial maximum vs today[J]. Quaternary Science Reviews, 1998, 17(4/5): 395−409. [15] Yasuda T, Asahara Y, Ichikawa R, et al. Distribution and transport processes of lithogenic material from the Amur River revealed by the Sr and Nd isotope ratios of sediments from the Sea of Okhotsk[J]. Progress in Oceanography, 2014, 126: 155−167. doi: 10.1016/j.pocean.2014.04.015 [16] Tessier A, Campbell P G C, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals[J]. Analytical Chemistry, 1979, 51(7): 844−851. doi: 10.1021/ac50043a017 [17] Quevauviller P, Rauret G, Muntau H, et al. Evaluation of a sequential extraction procedure for the determination of extractable trace metal contents in sediments[J]. Fresenius' Journal of Analytical Chemistry, 1994, 349(12): 808−814. doi: 10.1007/BF00323110 [18] 于增慧, 高玉花, 翟世奎, 等. 冲绳海槽中部沉积物中热液源组分的顺序淋滤萃取研究[J]. 中国科学: 地球科学, 2012, 55(4): 665−674.Yu Zenghui, Gao Yuhua, Zhai Shikui, et al. Resolving the hydrothermal signature by sequential leaching studies of sediments from the middle of the Okinawa Trough[J]. Science China Earth Sciences, 2012, 55(4): 665−674. [19] 邹亮, 韦刚健. 顺序提取法探讨沉积物中主量元素在不同相态的分配特征[J]. 海洋地质与第四纪地质, 2007, 27(2): 133−140.Zou Liang, Wei Gangjian. Distribution of major elements in sediment by sequential extraction procedures[J]. Marine Geology & Quaternary Geology, 2007, 27(2): 133−140. [20] 杨丽, 高爱国, 张延颇, 等. 西北冰洋表层沉积物中重金属的赋存形态研究[J]. 台湾海峡, 2012, 31(4): 451−458.Yang Li, Gao Aiguo, Zhang Yanpo, et al. Study on the speciation of heavy metals in the sediments of the western Arctic Ocean[J]. Journal of Oceanography in Taiwan Strait, 2012, 31(4): 451−458. [21] Piotrowski A M, Goldstein S L, Hemming S R, et al. Temporal relationships of carbon cycling and ocean circulation at glacial boundaries[J]. Science, 2005, 307(5717): 1933−1938. doi: 10.1126/science.1104883 [22] Piotrowski A M, Goldstein S L, Hemming S R, et al. Intensification and variability of ocean thermohaline circulation through the last deglaciation[J]. Earth and Planetary Science Letters, 2004, 225(1/2): 205−220. [23] 曹鹏, 石学法, 李巍然, 等. 安达曼海东南部海域表层沉积物稀土元素特征及其物源指示意义[J]. 海洋地质与第四纪地质, 2015, 35(5): 57−67.Cao Peng, Shi Xuefa, Li Weiran, et al. Rare earth element geochemistry of surface sediments in southeastern Andaman Sea and implications for provenance[J]. Marine Geology & Quaternary Geology, 2015, 35(5): 57−67. [24] 何连花, 张俊, 高晶晶, 等. 地质样品Sr和Nd同位素的化学分离方法改进[J]. 海洋科学进展, 2014, 32(1): 78−83. doi: 10.3969/j.issn.1671-6647.2014.01.009He Lianhua, Zhang Jun, Gao Jingjing, et al. Improvement of the method for chemical separations of Sr and Nd in geological samples[J]. Advances in Marine Science, 2014, 32(1): 78−83. doi: 10.3969/j.issn.1671-6647.2014.01.009 [25] Steiger R H, Jäger E. Subcommission on geochronology: convention on the use of decay constants in geo- and cosmochronology[J]. Earth and Planetary Science Letters, 1977, 36(3): 359−362. doi: 10.1016/0012-821X(77)90060-7 [26] Tanaka T, Togashi S, Kamioka H, et al. JNdi-1: a neodymium isotopic reference in consistency with LaJolla neodymium[J]. Chemical Geology, 2000, 168(3/4): 279−281. [27] Jacobsen S B, Wasserburg G J. Sm–Nd isotopic evolution of chondrites[J]. Earth and Planetary Science Letters, 1980, 50(1): 139−155. doi: 10.1016/0012-821X(80)90125-9 [28] Palmer M R, Elderfield H. Sr isotope composition of sea water over the past 75 Myr[J]. Nature, 1985, 314(6011): 526−528. doi: 10.1038/314526a0 [29] Henderson G M, Martel D J, O’Nions R K, et al. Evolution of seawater 87Sr86Sr over the last 400 ka: the absence of glacial/interglacial cycles[J]. Earth and Planetary Science Letters, 1994, 128(3/4): 643−651. [30] Mokadem F, Parkinson I J, Hathorne E C, et al. High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: limits on glacial–interglacial variations in continental weathering[J]. Earth and Planetary Science Letters, 2015, 415: 111−120. doi: 10.1016/j.jpgl.2015.01.036 [31] Du Jianghui, Haley B A, Mix A C. Neodymium isotopes in authigenic phases, bottom waters and detrital sediments in the Gulf of Alaska and their implications for paleo-circulation reconstruction[J]. Geochimica et Cosmochimica Acta, 2016, 193: 14−35. doi: 10.1016/j.gca.2016.08.005 [32] Molina-Kescher M, Frank M, Hathorne E C. Nd and Sr isotope compositions of different phases of surface sediments in the South Pacific: extraction of seawater signatures, boundary exchange, and detrital/dust provenance[J]. Geochemistry, Geophysics, Geosystems, 2014, 15(9): 3502−3520. doi: 10.1002/2014GC005443 [33] 赵葵东, 蒋少涌, 郑新源, 等. 海洋Nd同位素演化及古洋流循环示踪研究[J]. 地学前缘, 2009, 16(5): 160−171. doi: 10.3321/j.issn:1005-2321.2009.05.016Zhao Kuidong, Jiang Shaoyong, Zheng Xinyuan, et al. Nd isotope evolution of ocean waters and implications for paleo-ocean circulation[J]. Earth Science Frontiers, 2009, 16(5): 160−171. doi: 10.3321/j.issn:1005-2321.2009.05.016 [34] 吴琼, 刘志飞. Nd同位素方法应用于示踪古洋流的研究进展[J]. 地球科学进展, 2010, 25(2): 220−229.Wu Qiong, Liu Zhifei. Research advance in tracing evolution pattern of paleo-currents by using Nd isotpic composition[J]. Advances in Earth Science, 2010, 25(2): 220−229. [35] Tachikawa K, Arsouze T, Bayon G, et al. The large-scale evolution of neodymium isotopic composition in the global modern and Holocene ocean revealed from seawater and archive data[J]. Chemical Geology, 2017, 457: 131−148. doi: 10.1016/j.chemgeo.2017.03.018 [36] Frank M. Radiogenic isotopes: tracers of past ocean circulation and erosional input[J]. Reviews of Geophysics, 2002, 40(1): 1001. doi: 10.1029/2000RG000094 [37] Tachikawa K, Roy-Barman M, Michard A, et al. Neodymium isotopes in the Mediterranean Sea: comparison between seawater and sediment signals[J]. Geochimica et Cosmochimica Acta, 2004, 68(14): 3095−3106. doi: 10.1016/j.gca.2004.01.024 [38] Stumpf R, Frank M, Schönfeld J, et al. Late quaternary variability of Mediterranean outflow water from radiogenic Nd and Pb isotopes[J]. Quaternary Science Reviews, 2010, 29(19/20): 2462−2472. [39] Broecker W S, Gerard R, Ewing M, et al. Natural radiocarbon in the Atlantic Ocean[J]. Journal of Geophysical Research, 1960, 65(9): 2903−2931. doi: 10.1029/JZ065i009p02903 [40] Bertram C J, Elderfield H. The geochemical balance of the rare earth elements and neodymium isotopes in the oceans[J]. Geochimica et Cosmochimica Acta, 1993, 57(9): 1957−1986. doi: 10.1016/0016-7037(93)90087-D [41] Colin C, Turpin L, Bertaux J, et al. Erosional history of the Himalayan and Burman ranges during the last two glacial–interglacial cycles[J]. Earth and Planetary Science Letters, 1999, 171(4): 647−660. doi: 10.1016/S0012-821X(99)00184-3 [42] Taylor S R, McLennan S M. The Continental Crust: its Composition and Evolution: an Examination of the Geochemical Record Preserved in Sedimentary Rocks[M]. Oxford: Blackwell Scientific Publication, 1985: 312. [43] 姜学钧, 林学辉, 姚德, 等. 稀土元素在水成型海洋铁锰结壳中的富集特征及机制[J]. 中国科学: 地球科学, 2011, 54(2): 197−203.Jiang Xuejun, Lin Xuehui, Yao De, et al. Enrichment mechanisms of rare earth elements in marine hydrogenic ferromanganese crusts[J]. Science China Earth Sciences, 2011, 54(2): 197−203. [44] Hein J R, Koschinsky A, Bau M, et al. Cobalt-rich ferromanganese crusts in the Pacific[M]//Cronan D S. Handbook of Marine Mineral Deposits. Boca Raton: CRC Press, 1999: 239−279. -




 下载:
下载:



