Response of redox sensitive elements to changes of sedimentary environment in core sediments of seasonal low-oxygen zone in East China Sea
-
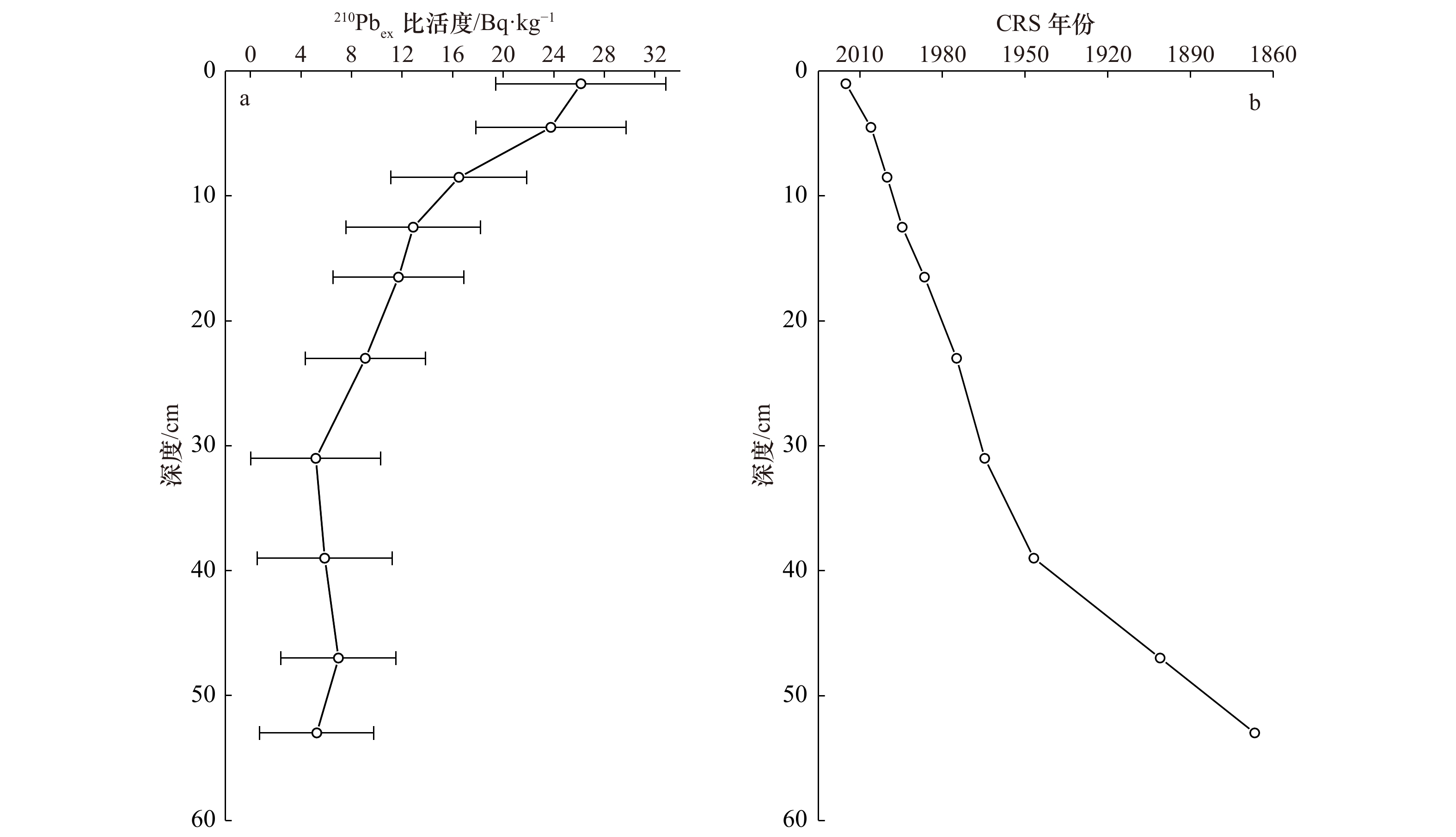
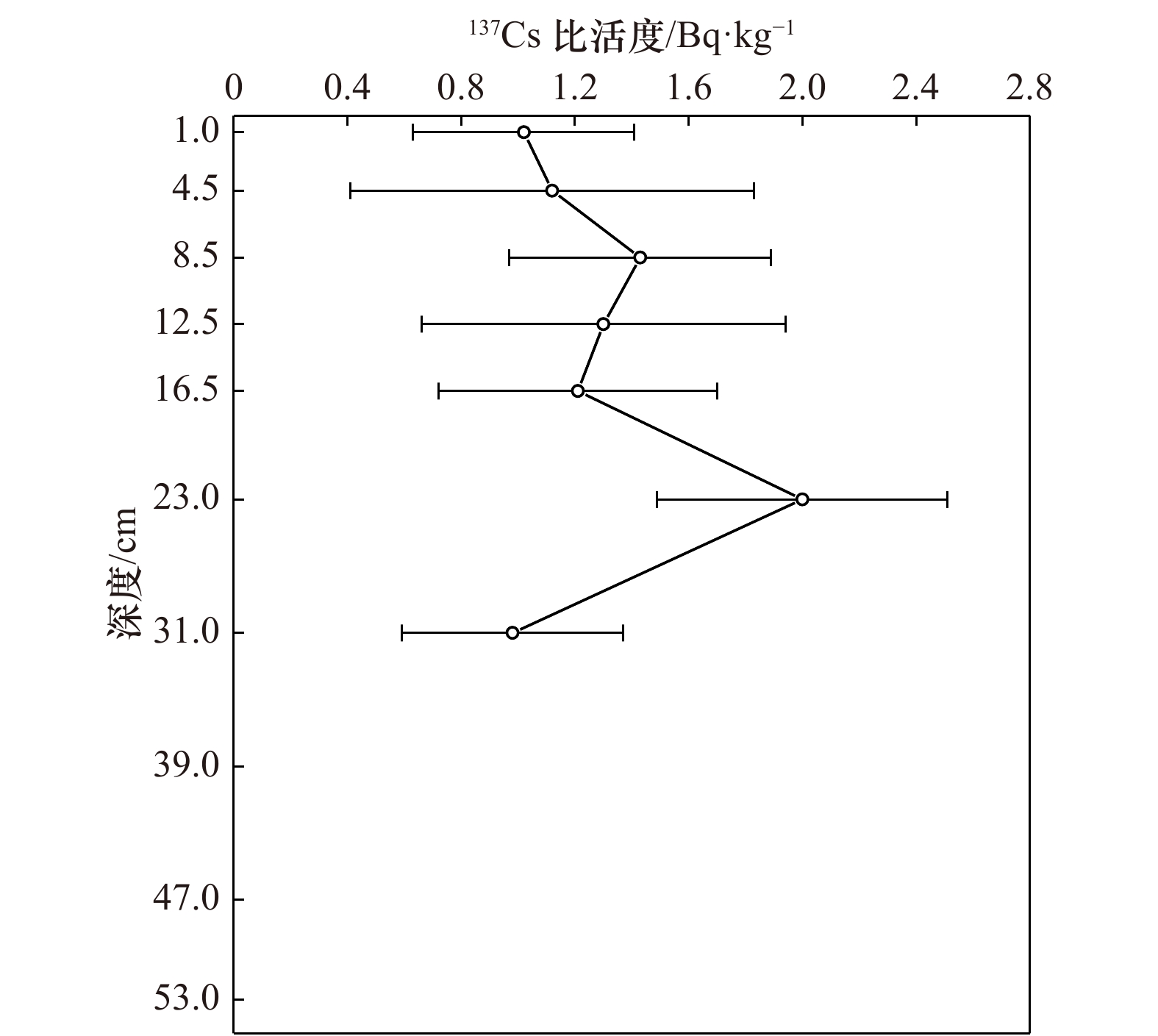
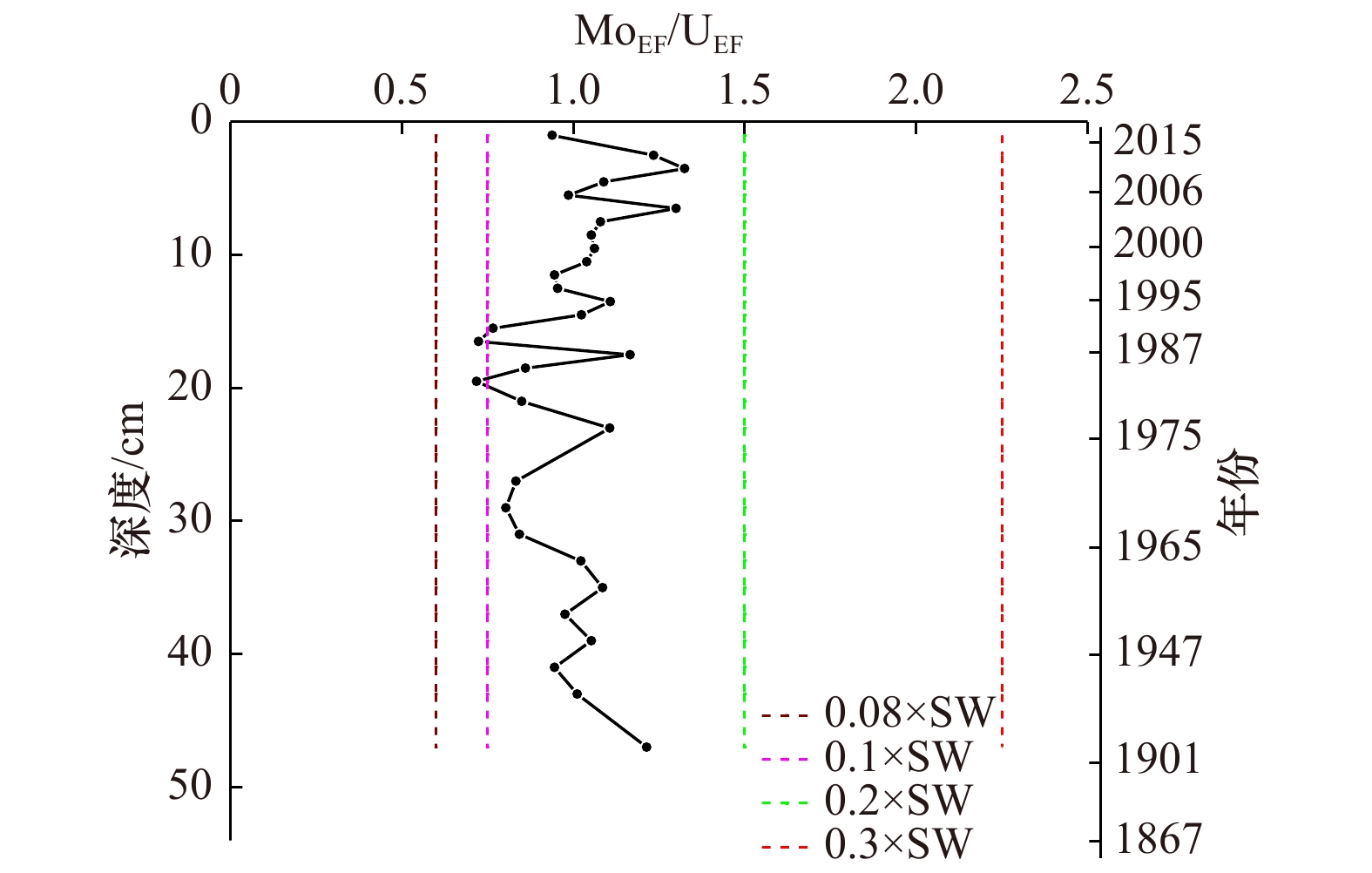
摘要: 氧化还原敏感元素(Redox Sensitive Elements,RSE)如V、Cr、Mo、U等,通常在氧化条件下呈溶解态,在还原沉积环境中除Fe、Mn外,RSE被还原成低价态转移至沉积物中富集积累,因此可以利用氧化还原敏感元素在沉积物中的富集情况反演沉积环境的氧化还原状况。本文通过研究东海内陆架季节性低氧海区Zb7沉积柱中氧化还原敏感元素V、Cr、Ni、Cu、Zn、Mo、U的垂直分布、富集特征和比值,探究沉积环境氧化还原状况;发现RSE/Al和富集系数自1978年以来呈增加的趋势,但自2009年开始有所降低,整体RSE富集系数均小于3,未见明显富集。RSE比值V/Cr<2、Ni/Co<5、U/Th<0.75、0.25<(Cu+Mo)/Zn<0.55,以及MoEF/UEF比值主要分布在0.08~0.3倍海水Mo/U值之间,均指示氧化的沉积环境。RSE/Al与Fe/Al、Mn/Al具有显著的相关性,表明RSE在剔除陆源碎屑输入后,主要通过与Fe、Mn氧化物结合进入沉积物,也指示氧化的沉积环境。研究结果与该区域溶解氧历史数据反映的季节性低氧结果不一致,可能与RSE在夏季季节性低氧时,沉积物中的富集信号在秋冬季溶氧水平恢复后缺失有关。尽管RSE不能有效指示东海季节性低氧环境,但Zb7沉积柱RSE在1978年后富集程度的增加以及2011年后的降低,在一定程度上反映了该区域自1978年后季节性低氧程度加重,2009年后又有所缓解的变化趋势。Abstract: Redox sensitive elements (Redox Sensitive Elements, RSE) such as V, Cr, Mo and U, et al. are dissolved under oxidation conditions. They are reduced to low-valent state under reducing sedimentary environment and transferred to sediments for enrichment and accumulation. Therefore, the enrichment of redox sensitive elements in sediments can be used to invert the redox status of sedimentary environment. In this paper, the vertical distribution, enrichment characteristics and ratios of redox sensitive elements V, Cr, Ni, Cu, Zn, Mo and U in the Zb7 core sediment in the seasonal low-oxygen sea area of East China Sea were studied. It was found that RSE/Al and enrichment coefficient had increased since 1978, but had decreased since 2009. The overall RSE enrichment coefficient was less than 3, and no obvious enrichment had been observed. RSE ratios V/Cr<2, Ni/Co<5, U/Th<0.75, 0.25<(Cu+Mo)/Zn<0.55 and MoEF/UEF ratios were mainly distributed between 0.08 and 0.3 times of seawater Mo/U ratios, indicating the oxidized sedimentary environment. RSE/Al was significantly correlated with Fe/Al and Mn/Al, which indicated that RSE entered sediments mainly by combining with Fe and Mn oxides after removing terrigenous debris inputs, and also indicated the oxidized sedimentary environment. The results were inconsistent with the results of seasonal hypoxia reflected by the historical data of dissolved oxygen in this region. It may be related to the absence of RSE enrichment signals in the sediments during seasonal hypoxia in summer after the recovery of dissolved oxygen levels in autumn and winter. Although RSE can not effectively indicate the seasonal hypoxic environment in the East China Sea, the increase of RSE enrichment in Zb7 after 1978 and the decrease after 2009 reflected to some extent the trend of seasonal hypoxia aggravating in the region since 1978 and easing after 2009.
-
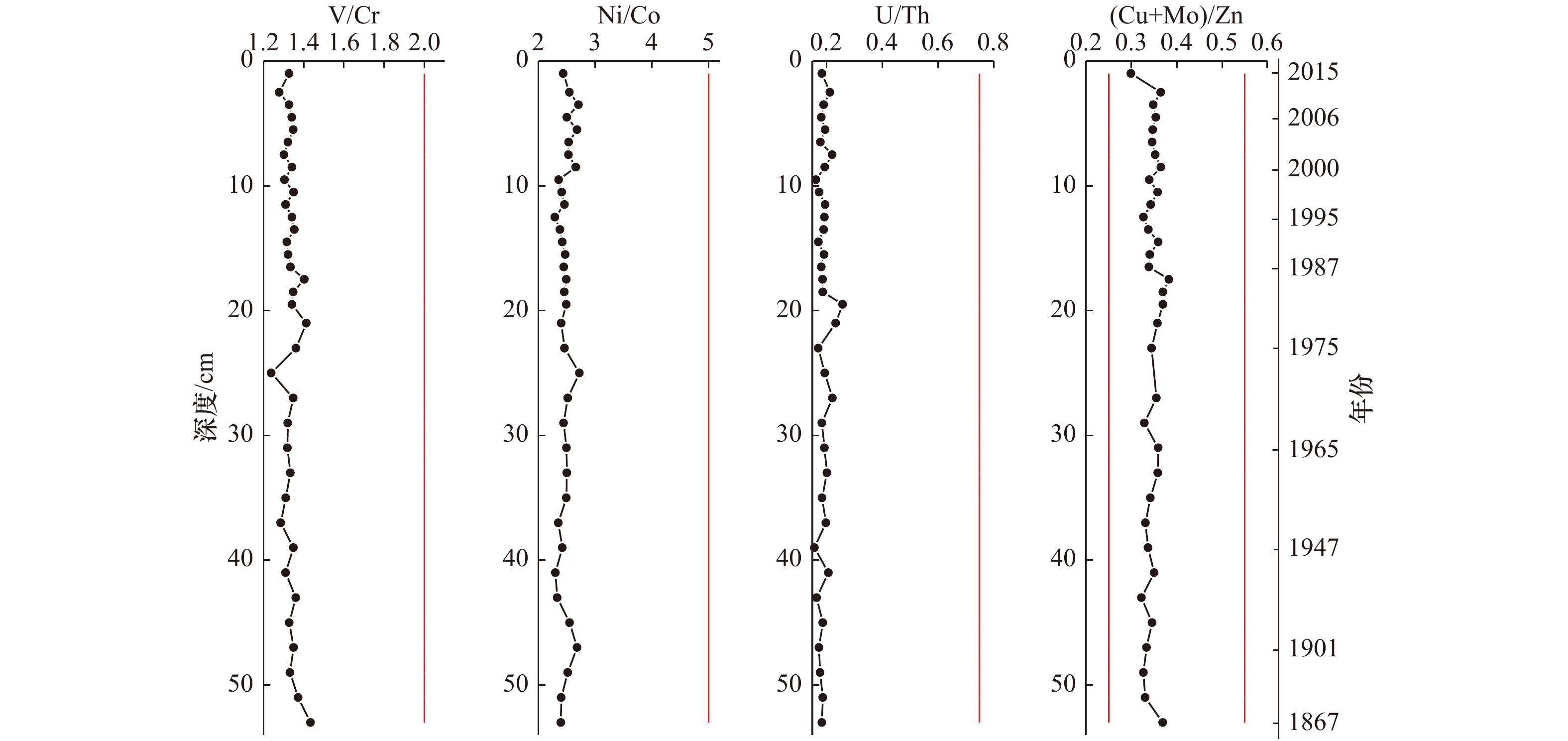
图 8 Zb7柱状沉积样品RSE比值垂直分布
图中红色竖线分别代表氧化环境-次氧化环境的阈值,从左到右,依次代表V/Cr=2,N/Co=5,U/Th=0.75,(Cu+Mo)/Zn=0.25,0.55
Fig. 8 The vertical distribution diagram of RSE’ ratios of Zb7 core sediments
The red vertical lines in the figure represent the threshold values of the RSE ratio in the oxic-suboxic sedimentary environment respectively, which successively represent V/Cr=2,N/Co=5,U/Th=0.75,(Cu+Mo)/Zn=0.25,0.55 from left to right
表 1 Zb7站位附近水域近年来低氧状况统计
Tab. 1 Statistics of low oxygen conditions in adjacent waters of Zb7 in recent years
时间 面积/km2 低氧位置 氧最小值/mg·L−1 氧平均值/mg·L−1 数据来源 1958年9月 16 400 29.23°N 122.47°E 0.73 2.51 参考文献[10] 1958年10月 2 370 29.73°N 122.76°E 1.83 2.51 参考文献[10] 1975年10月 2 330 30.88°N 123.50°E 2.07 2.72 参考文献[10] 1980年10月 528 29.01°N 123.50°E 2.59 2.85 参考文献[10] 1999年8月 13 700 30.83°N 122.75°E 1.00 2.51 参考文献[8] 2003年6月 8 370 30.95°N 122.71°E 1.00 2.56 参考文献[18] 2006年10月 11 000 29.07°N 123.67°E 1.91 2.88 参考文献[8] 2015年6月 − 30.0°N 123.0°E 3.06 − 参考文献[6] 2015年8月 − 29.14°N 122.47°E 1.92 − 参考文献[23] 表 2 微波消解程序
Tab. 2 Microwave digestion procedure
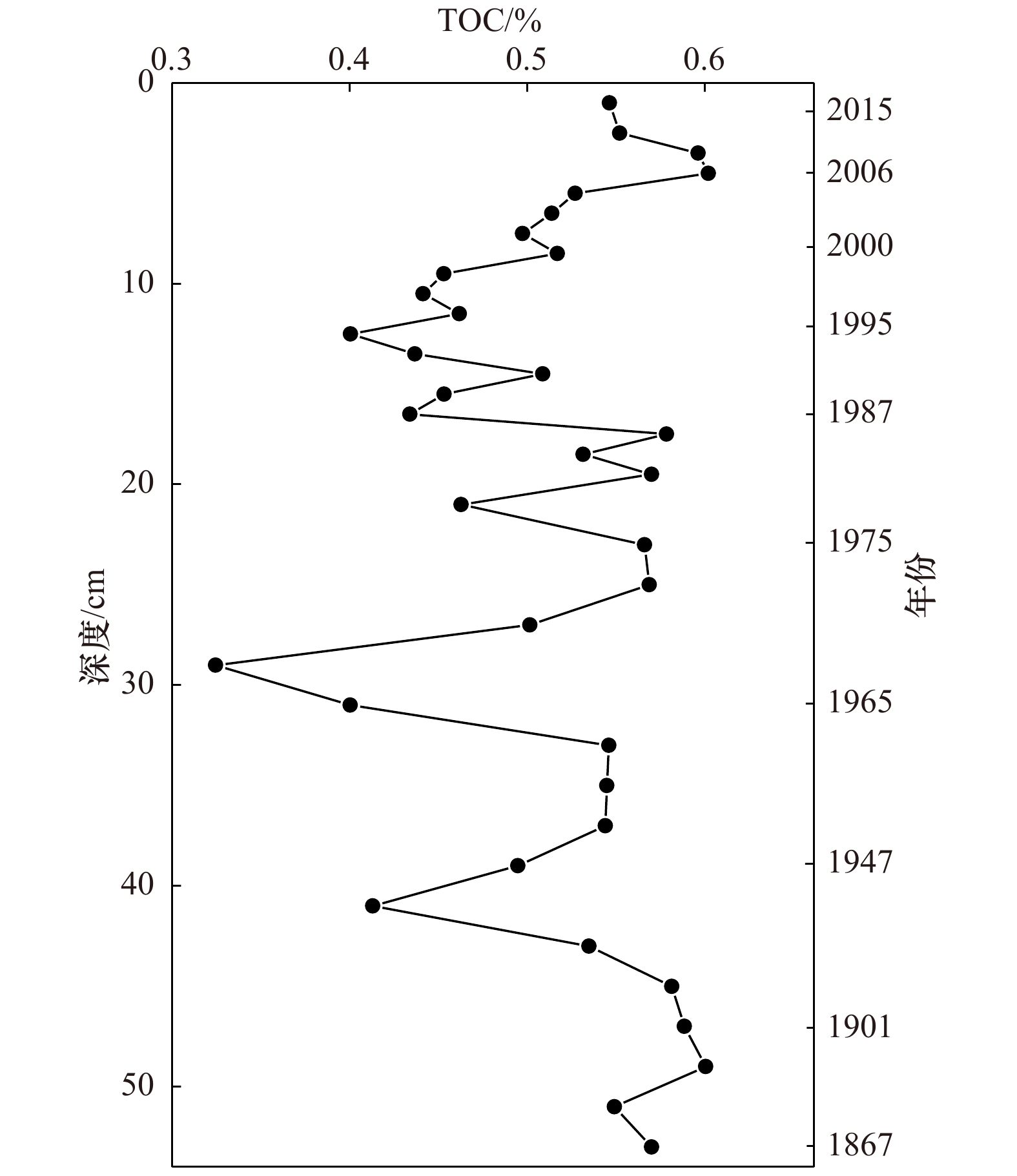
功率/W 升温时间/min 升至温度/℃ 保持时间/min 1 600 3 140 3 1 600 3 160 5 1 600 3 180 30 表 3 RSE/Al与Fe/Al、Mn/Al和TOC的相对变化关系
Tab. 3 Relative variations of RSE/Al with respect to Fe/Al、Mn/Al and TOC
V/Al Cr/Al Ni/Al Cu/Al Zn/Al Mo/Al U/Al TOC 0.011 −0.008 0.164 0.209 0.154 0.341 −0.080 Fe/Al 0.826** 0.779** 0.808** 0.832** 0.863** 0.726* 0.644** Mn/Al 0.887** 0.856** 0.824** 0.847** 0.885** 0.694* 0.721** 注:*代表显著性水平p<0.05(双尾检验);**代表显著性水平p<0.01(双尾检验)。 表 4 Zb7柱状沉积物RSE与粒度的相关性分析
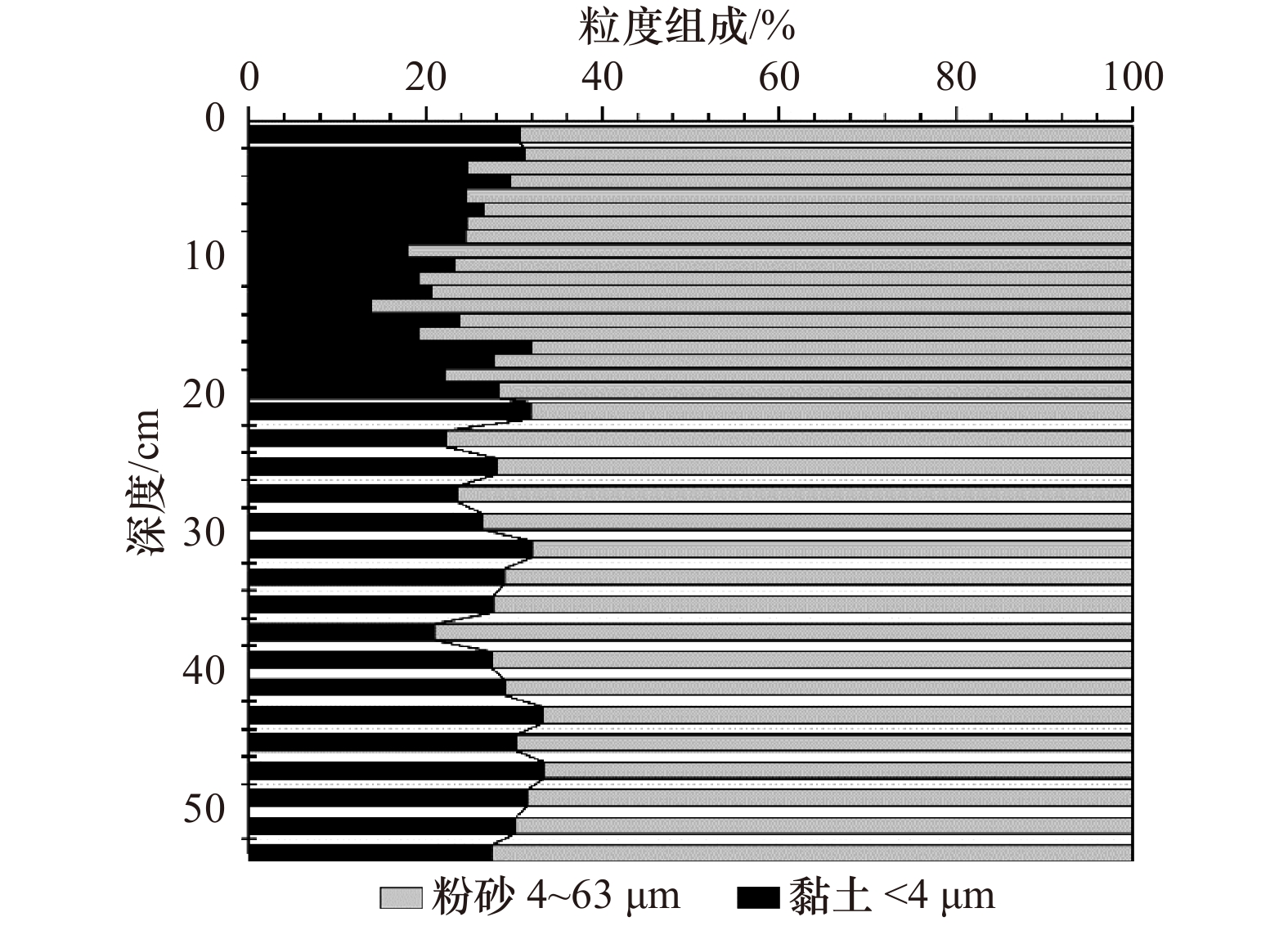
Tab. 4 Correlation analysis of RSE and grain sizes of Zb7 core sediments
<1 μm 1~2 μm 2~4 μm <4 μm 4~8 μm 8~16/μm 16~63 μm 4~63 μm D50 Dav Al 0.070 0.480** 0.284 0.381* 0.321 0.024 −0.270 −0.381* −0.331 −0.289 Fe −0.075 0.375* 0.357* 0.386* 0.503** −0.19 −0.205 −0.386* −0.425** −0.287 Mn −0.086 0.414* 0.156 0.255 0.325 0.053 −0.244 −0.255 −0.293 −0.267 V −0.178 0.145 0.155 0.152 0.367* −0.407* 0.072 −0.152 −0.259 −0.038 Cr −0.272 0.085 0.116 0.097 0.345* −0.394* 0.095 −0.097 −0.212 −0.005 Ni −0.305 −0.003 0.079 0.037 0.301 −0.391* 0.132 −0.037 −0.15 0.032 Cu −0.283 0.055 0.041 0.032 0.253 −0.299 0.092 −0.032 −0.153 0.002 Zn −0.304 0.032 0.105 0.068 0.326 −0.269 0.036 −0.068 −0.187 −0.037 Mo −0.092 0.120 0.217 0.193 0.397* −0.195 −0.086 −0.193 −0.285 −0.187 U −0.082 0.326 0.360* 0.370* 0.427** −0.192 −0.174 −0.370* −0.432** −0.276 Th −0.087 0.331* 0.353* 0.366* 0.493** −0.075 −0.265 −0.366* −0.457** −0.350* TOC −0.113 0.272 0.303 0.307 0.504** −0.333* −0.084 −0.307 −0.371* −0.192 注:*代表显著性水平p<0.05(双尾检验);**代表显著性水平p<0.01(双尾检验)。 表 5 RSE比值对氧化还原环境的指示
Tab. 5 The indication of RSE’ ratios on redox environment
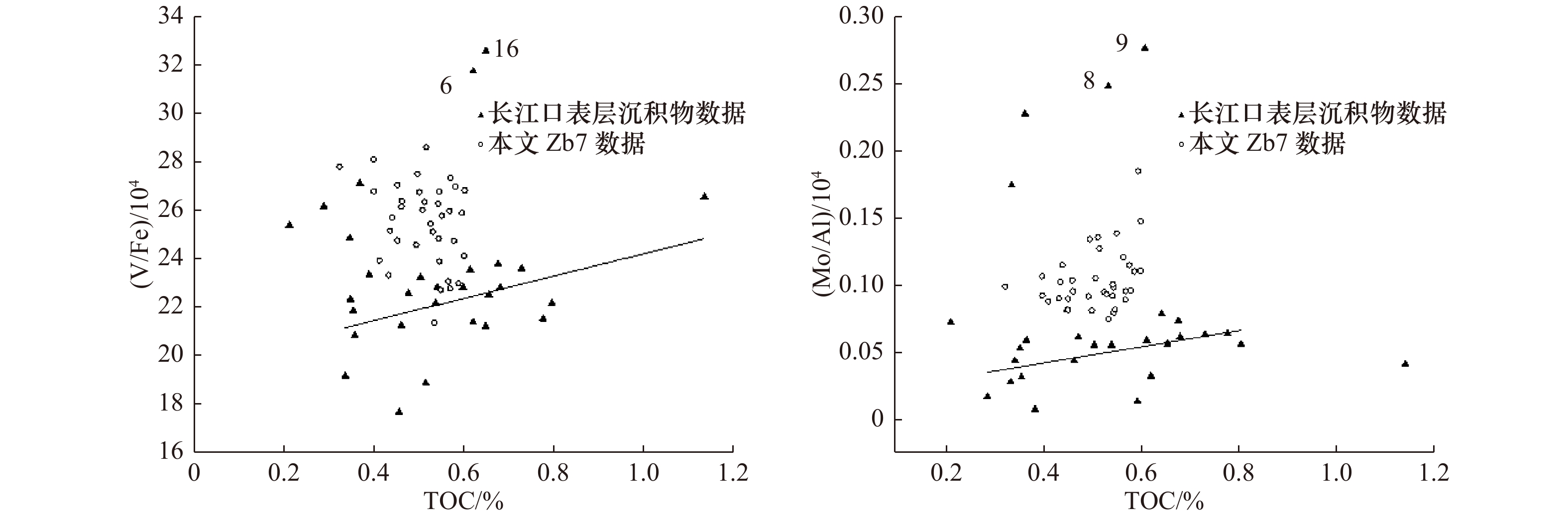
表 6 Zb7沉积柱与长江口及其邻近海域表层沉积物中RSE/Al的对比
Tab. 6 Comparisons of RSE /Al between Zb7 core sediments and surface sediments of the Changjiang River Estuary and its adjacent waters
采样位置 (V/Al)/103 (Mo/Al)/106 (U/Al)/105 参考文献 长江口外低氧区8站(31.15°N,122.56°E) 1.43 47.53 3.18 [19] 长江口外低氧区9站(31.02°N,122.62°E) 1.50 51.79 3.10 [19] 长江口外低氧区16站(30.94°N,122.72°E) 2.36 10.32 2.90 [19] 长江口外溶解氧正常区26站(29.98°N,122.74°E) 1.38 10.26 3.18 [19] 长江口外溶解氧正常区29站(29.31°N,122.18°E) 1.27 9.39 3.50 [19] Zb7沉积柱RSE/Al最高值 1.72 18.51 5.31 本研究 Zb7沉积柱RSE/Al最低值 1.04 7.48 2.81 本研究 -
[1] 赵晨英. 乳山湾近海与黄渤海溶解氧、有机碳、氮和磷的循环与收支的关键过程研究[D]. 青岛: 国家海洋局第一海洋研究所, 2017.Zhao Chenying. Controlling processes of dissolved oxygen, organic carbon, nitrogen and phosphorus cycles and budgets in the coastal area of Rushan Bay and Bohai and Yellow Seas[D]. Qingdao: First Institute of Oceanography, SOA, 2017. [2] 韦钦胜, 王守强, 臧家业, 等. 海洋低氧现象的研究及相关问题初探[J]. 海洋开发与管理, 2009, 26(6): 54−59. doi: 10.3969/j.issn.1005-9857.2009.06.012Wei Qinsheng, Wang Shouqiang, Zang Jiaye, et al. Research on marine hypoxia phenomenon and some related issues[J]. Ocean Development and Management, 2009, 26(6): 54−59. doi: 10.3969/j.issn.1005-9857.2009.06.012 [3] Stramma L, Johnson G C, Sprintall J, et al. Expanding oxygen-minimum zones in the tropical oceans[J]. Science, 2008, 320(5876): 655−658. doi: 10.1126/science.1153847 [4] Wong G T F, Gong G C, Liu K K, et al. “Excess nitrate” in the East China Sea[J]. Estuarine, Coastal and Shelf Science, 1998, 46(3): 411−418. doi: 10.1006/ecss.1997.0287 [5] Zhou Mingjiang, Shen Zhiliang, Yu Rencheng. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River[J]. Continental Shelf Research, 2008, 28(12): 1483−1489. doi: 10.1016/j.csr.2007.02.009 [6] 刘军, 臧家业, 冉祥滨, 等. 长江口低氧区沉积物中磷的形态及其环境意义[J]. 环境科学, 2017, 38(8): 3243−3253.Liu Jun, Zang Jiaye, Ran Xiangbin, et al. Sedimentary phosphorus speciation in the coastal hypoxic area of Changjiang Estuary and its environmental significance[J]. Environmental Science, 2017, 38(8): 3243−3253. [7] 刘志国, 徐韧, 刘材材, 等. 长江口外低氧区特征及其影响研究[J]. 海洋通报, 2012, 31(5): 588−593.Liu Zhiguo, Xu Ren, Liu Caicai, et al. Characters of hypoxia area off the Yangtze River Estuary and its influence[J]. Marine Science Bulletin, 2012, 31(5): 588−593. [8] 李道季, 张经, 黄大吉, 等. 长江口外氧的亏损[J]. 中国科学: D辑, 2002, 32(8): 686−694. doi: 10.3321/j.issn:1006-9267.2002.08.009Li Daoji, Zhang Jing, Huang Daji, et al. Oxygen depletion off the Changjiang (Yangtze River) estuary[J]. Science in China : Series D, 2002, 32(8): 686−694. doi: 10.3321/j.issn:1006-9267.2002.08.009 [9] 张哲, 张志锋, 韩庚辰, 等. 长江口外低氧区时空变化特征及形成、变化机制初步探究[J]. 海洋环境科学, 2012, 31(4): 469−473. doi: 10.3969/j.issn.1007-6336.2012.04.002Zhang Zhe, Zhang Zhifeng, Han Gengchen, et al. Spatio-temporal variation, formation and transformation of the hypoxia area off the Yangtze River estuary[J]. Marine Environmental Science, 2012, 31(4): 469−473. doi: 10.3969/j.issn.1007-6336.2012.04.002 [10] 宋国栋. 东海溶解氧气候态分布及海洋学应用研究[D]. 青岛: 中国海洋大学, 2008.Song Guodong. Climatological parameters distributions of dissolved oxygen in the East China Sea and its application in the oceanography[D]. Qingdao: Ocean University of China, 2008. [11] Crusius J, Calvert S, Pedersen T, et al. Rhenium and molybdenum enrichments in sediments as indicators of oxic, suboxic and sulfidic conditions of deposition[J]. Earth and Planetary Science Letters, 1996, 145(1/4): 65−78. [12] 于宇, 宋金明, 李学刚, 等. 沉积物微量金属元素在重建水体环境变化中的意义[J]. 地质论评, 2012, 58(5): 911−922. doi: 10.3969/j.issn.0371-5736.2012.05.013Yu Yu, Song Jinming, Li Xuegang, et al. Significance of sedimentary trace metals in reconstructing the aquatic environmental Changes[J]. Geological Review, 2012, 58(5): 911−922. doi: 10.3969/j.issn.0371-5736.2012.05.013 [13] 宋金明, 李学刚. 海洋沉积物/颗粒物在生源要素循环中的作用及生态学功能[J]. 海洋学报, 2018, 40(10): 1−13.Song Jinming, Li Xuegang. Ecological functions and biogenic element cycling roles of marine sediment/particles[J]. Haiyang Xuebao, 2018, 40(10): 1−13. [14] Algeo T J, Tribovillard N. Environmental analysis of paleoceanographic systems based on molybdenum-uranium covariation[J]. Chemical Geology, 2009, 268(3/4): 211−225. [15] Acharya S S, Panigrahi M K, Gupta A K, et al. Response of trace metal redox proxies in continental shelf environment: the Eastern Arabian Sea scenario[J]. Continental Shelf Research, 2015, 106: 70−84. doi: 10.1016/j.csr.2015.07.008 [16] Algeo T J, Maynard J B. Trace-element behavior and redox facies in core shales of upper pennsylvanian kansas-type cyclothems[J]. Chemical Geology, 2004, 206(3/4): 289−318. [17] Colodner D, Edmond J, Boyle E. Rhenium in the Black Sea: comparison with molybdenum and uranium[J]. Earth and Planetary Science Letters, 1995, 131(1/2): 1−15. [18] 许淑梅. 长江口外缺氧区及其邻近海域氧化还原敏感性元素的分布规律及环境指示意义[D]. 青岛: 中国海洋大学, 2005.Xu Shumei. The distribution and environmental significance of redox sensitive elements in the hypoxia zone of the Changjiang Estuary and its Contiguous area[D]. Qingdao: Ocean University of China, 2008. [19] 许淑梅, 翟世奎, 张爱滨, 等. 长江口及其邻近海域表层沉积物中氧化还原敏感性微量元素的环境指示意义[J]. 沉积学报, 2007, 25(5): 759−766. doi: 10.3969/j.issn.1000-0550.2007.05.015Xu Shumei, Zhai Shikui, Zhang Aibin, et al. Distribution and environment significance of redox sensitive trace elements of the Changjiang estuary hypoxia zone and its contiguous sea area[J]. Acta Sedimentologica Sinica, 2007, 25(5): 759−766. doi: 10.3969/j.issn.1000-0550.2007.05.015 [20] 冯旭文. 长江口百年来底层水体季节性缺氧在沉积物中的记录[D]. 杭州: 浙江大学, 2009.Feng Xuwen. Sedimentary records of hypoxia in the Changjiang Estuary over last 100 years[D]. Hangzhou: Zhejiang University, 2009. [21] 冯旭文, 金翔龙, 章伟艳, 等. 长江口外缺氧区柱样沉积物元素的分布及其百年沉积环境效应[J]. 海洋地质与第四纪地质, 2009, 29(2): 25−32.Feng Xuwen, Jin Xianglong, Zhang Weiyan, et al. Variation of elements in sediments from the hypoxia zone of the Yangtze estuary and its response to sedimentary environment over the last 100 years[J]. Marine Geology & Quaternary Geology, 2009, 29(2): 25−32. [22] 郭伟. 东海赤潮区水体缺氧状况的沉积记录分析[D]. 青岛: 中国科学院海洋研究所, 2013.Guo Wei. Sedimentary records of hypoxia status in the red-tide zone of the East Sea[D]. Qingdao: The Institute of Oceanology, Chinese Academy of Sciences, 2013. [23] Chi Lianbao, Song Xiuxian, Yuan Yongquan, et al. Distribution and key influential factors of dissolved oxygen off the Changjiang river estuary (CRE) and its adjacent waters in China[J]. Marine Pollution Bulletin, 2017, 125(1/2): 440−450. [24] 黄思静. 用Excel计算沉积物粒度分布参数[J]. 成都理工学院学报, 1999, 26(2): 195−198.Huang Sijing. Calculation of grain size distribution parameters of sediments by microsoft Excel[J]. Journal of Chengdu University of Technology, 1999, 26(2): 195−198. [25] Piper D Z, Calvert S E. A marine biogeochemical perspective on black shale deposition[J]. Earth-Science Reviews, 2009, 95(1/2): 63−96. [26] Abrahim G M S, Parker R J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand[J]. Environmental Monitoring and Assessment, 2008, 136(1/3): 227−238. [27] Shepard F P. Nomenclature based on sand-silt-clay ratios[J]. Journal of Sedimentary Petrology, 1954, 24(3): 151−158. [28] Tribovillard N, Algeo T J, Lyons T, et al. Trace metals as paleoredox and paleoproductivity proxies: an update[J]. Chemical Geology, 2006, 232(1/2): 12−32. [29] Turgeon S, Brumsack H J. Anoxic vs dysoxic events reflected in sediment geochemistry during the Cenomanian-Turonian Boundary Event (Cretaceous) in the Umbria-Marche Basin of central Italy[J]. Chemical Geology, 2006, 234(3/4): 321−339. [30] Morford J L, Emerson S. The geochemistry of redox sensitive trace metals in sediments[J]. Geochimica et Cosmochimica Acta, 1999, 63(11/12): 1735−1750. [31] Calvert S E, Pedersen T F. Geochemistry of recent oxic and anoxic marine sediments: implications for the geological record[J]. Marine Geology, 1993, 113(1/2): 67−88. [32] Jones B, Manning D A C. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones[J]. Chemical Geology, 1994, 111(1/4): 111−129. [33] Hallberg R O. A geochemical method for investigation of paleoredox conditions in sediments[J]. Ambio Special Report, 1976(4): 139−147. [34] 汤冬杰, 史晓颖, 赵相宽, 等. Mo-U共变作为古沉积环境氧化还原条件分析的重要指标——进展、问题与展望[J]. 现代地质, 2015, 29(1): 1−13. doi: 10.3969/j.issn.1000-8527.2015.01.001Tang Dongjie, Shi Xiaoying, Zhao Xiangkuan, et al. Mo-U covariation as an important proxy for sedimentary environment redox conditions—progress, problems and Prospects[J]. Geoscience, 2015, 29(1): 1−13. doi: 10.3969/j.issn.1000-8527.2015.01.001 [35] Shaheen S M, Ali R A, Abowaly M E, et al. Assessing the mobilization of As, Cr, Mo, and Se in Egyptian lacustrine and calcareous soils using sequential extraction and biogeochemical microcosm techniques[J]. Journal of Geochemical Exploration, 2018, 191: 28−42. doi: 10.1016/j.gexplo.2018.05.003 [36] McLennan S M. Relationships between the trace element composition of sedimentary rocks and upper continental crust[J]. Geochemistry, Geophysics, Geosystems, 2001, 2(4): 2000GC000109. [37] Berner R A, Raiswell R. Burial of organic carbon and pyrite sulfur in sediments over phanerozoic time: a new theory[J]. Geochimica et Cosmochimica Acta, 1983, 47(5): 855−862. doi: 10.1016/0016-7037(83)90151-5 [38] 张岩松, 章飞军, 郭学武, 等. 东海秋季典型站位沉降颗粒物通量[J]. 海洋与湖沼, 2006, 37(1): 28−34. doi: 10.3321/j.issn:0029-814X.2006.01.005Zhang Yansong, Zhang Feijun, Guo Xuewu, et al. Autumn flux of particle settling observed at three representative stations in East China Sea[J]. Oceanologia et Limnologia Sinica, 2006, 37(1): 28−34. doi: 10.3321/j.issn:0029-814X.2006.01.005 -





 下载:
下载: