Self-assembled membrane injection mass spectrometry system and its application on the study of dissimilatory nitrate reduction in sandy sediments
-
摘要: 沉积物中的异化硝酸盐还原过程对于海洋氮循环起着至关重要的作用。基于15N标记的培养技术是目前测定沉积物异化硝酸盐还原的主要手段。准确快速测定15N标记的产物(29N2、 30N2)是量化异化硝酸盐还原各个过程速率的关键。本研究自行组装膜进样质谱系统用于29N2和30N2的测定,对其测量条件进行了优化。结果表明,进样蠕动泵进样流速0.80 mL/min,进样时间3~3.5 min,恒温槽温度20~25°C,同时铜还原炉温度在300~600°C的条件下,29N2/28N2和30N2/28N2的测试精密度分别可以控制在0.1%和1%以内,比较适合29N2和30N2的测定。利用自组装的膜进样质谱系统结合15N标记的培养技术研究了青岛石老人沙滩沉积物中的异化硝酸盐还原过程。石老人沙滩沉积物不存在将硝酸盐完全还原为氮气好氧的反硝化。厌氧铵氧化、厌氧反硝化和异化硝酸盐还原为铵(Dissimilatory Nitrate Reduction to Ammonium, DNRA)的潜在速率 (以湿沉积物N计)分别为(0.05±0.01) nmol /(cm3·h),(2.32±0.21) nmol /(cm3·h)和(1.02±0.15) nmol /(cm3·h)。厌氧反硝化是硝酸盐异化还原主要的贡献者,其比例接近70%,其次是DNRA,比例可达30%,而厌氧铵氧化的贡献最低,仅为1%。在N2产生过程中,主要贡献者是反硝化,厌氧铵氧化的贡献仅为2%。Abstract: Dissimilatory nitrate reduction processes in sediments play a crucial role in marine nitrogen cycle. The most popular method to determine the rates of different dissimilatory nitrate reduction processes is the 15N labeled technique. Therefore, accurate and rapid determination the concentration of 15N-labeled products, such as 29N2 and 30N2, is the key to quantify the rate of each dissimilatory nitrate reduction process. In this study, we set up a membrane injection mass spectrometry (MIMS) and optimize the operating condition of the MIMS for the determination of 29N2 and 30N2. The optimization experiment results indicated that, when the peristaltic pump for sampling flow rate is 0.80 mL/min, the sampling time is 3~3.5 min, the thermostat water bath temperature is 20~25℃, and the copper reduction furnace temperature is 300~600℃, the precision (expressed in coefficient of variation) of the measured 29N2/28N2 and 30N2/28N2 can be controlled less than 0.1% and 1%, respectively. We used the self-assembled MIMS and combined the 15N labeling technique to study the dissimilatory nitrate reduction processes in the sandy sediment of the Shilaoren beach in Qingdao. There is no significant aerobic denitrification in the Shilaoren sand that can completely reduce nitrate to N2. The potential rates of anammox, anaerobic denitrification and dissimilatory nitrate reduction to ammonium (DNRA) are (0.05±0.01) nmol/(cm3·h), (2.32±0.21) nmol/(cm3·h) and (1.02±0.15) nmol/(cm3·h) (N, wet sed.), respectively. Anaerobic denitrification is the major contributor to nitrate dissimilatory reduction, with a ratio of nearly 70%, followed by DNRA, with a ratio of up to 30%, while anammox has the lowest contribution of only 1%. In the N2 production, the main contributor is anaerobic denitrification, and the contribution of anammox is only 2%.
-
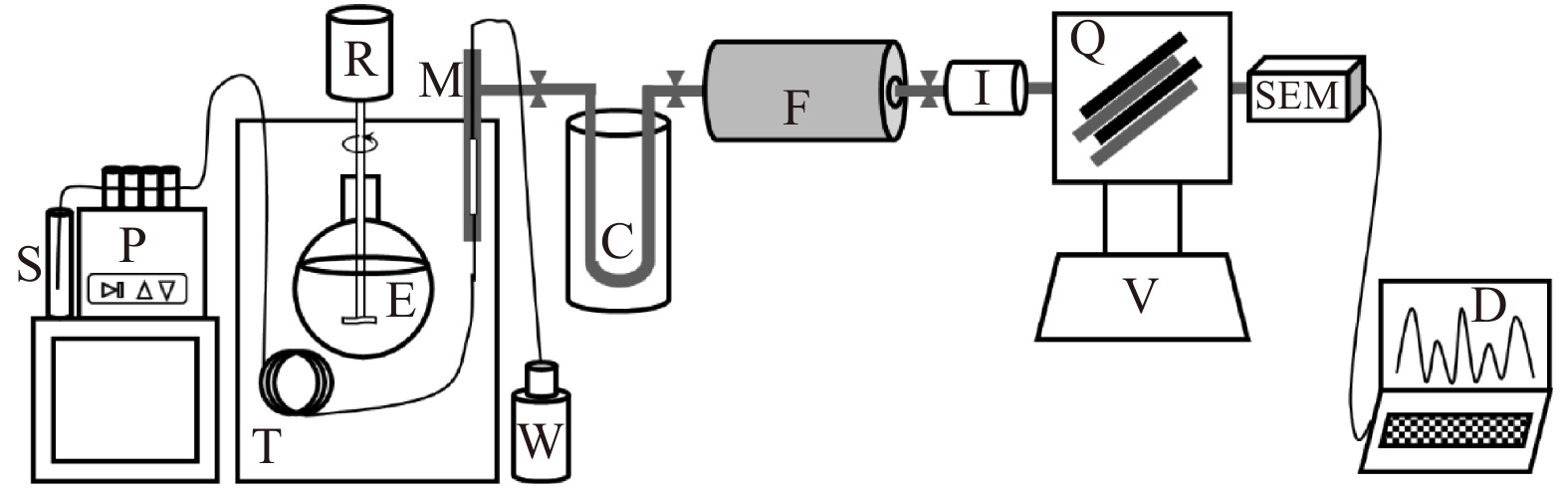
图 1 自组装膜进样质谱系统示意图
从左到右的主要部件依次为:样品瓶(S),进样蠕动泵(P),恒温水浴槽(T),标准样品制作系统(R-E),膜进样器(M),废液回收瓶(W),冷阱(C),铜还原炉(F,内含一根装有还原铜丝的石英管),抽真空系统(V),离子源(I),四极杆质量分析器(Q),二次电子倍增检测器(SEM),数据处理系统(D)
Fig. 1 Schematic diagram of self-assembled membrane injection mass spectrometry system
The main components from left to right are: sample vial (S), injection peristaltic pump (P), constant temperature water bath (T), standard sample preparation system (R-E), membrane injector (M), waste recovery bottle (W), cold trap (C), copper reduction furnace (F, containing a quartz tube with reduced copper wire), vacuum system (V), Ion source (I), quadrupole mass analyzer (Q), secondary electron multiplier(SEM), data processing system (D)
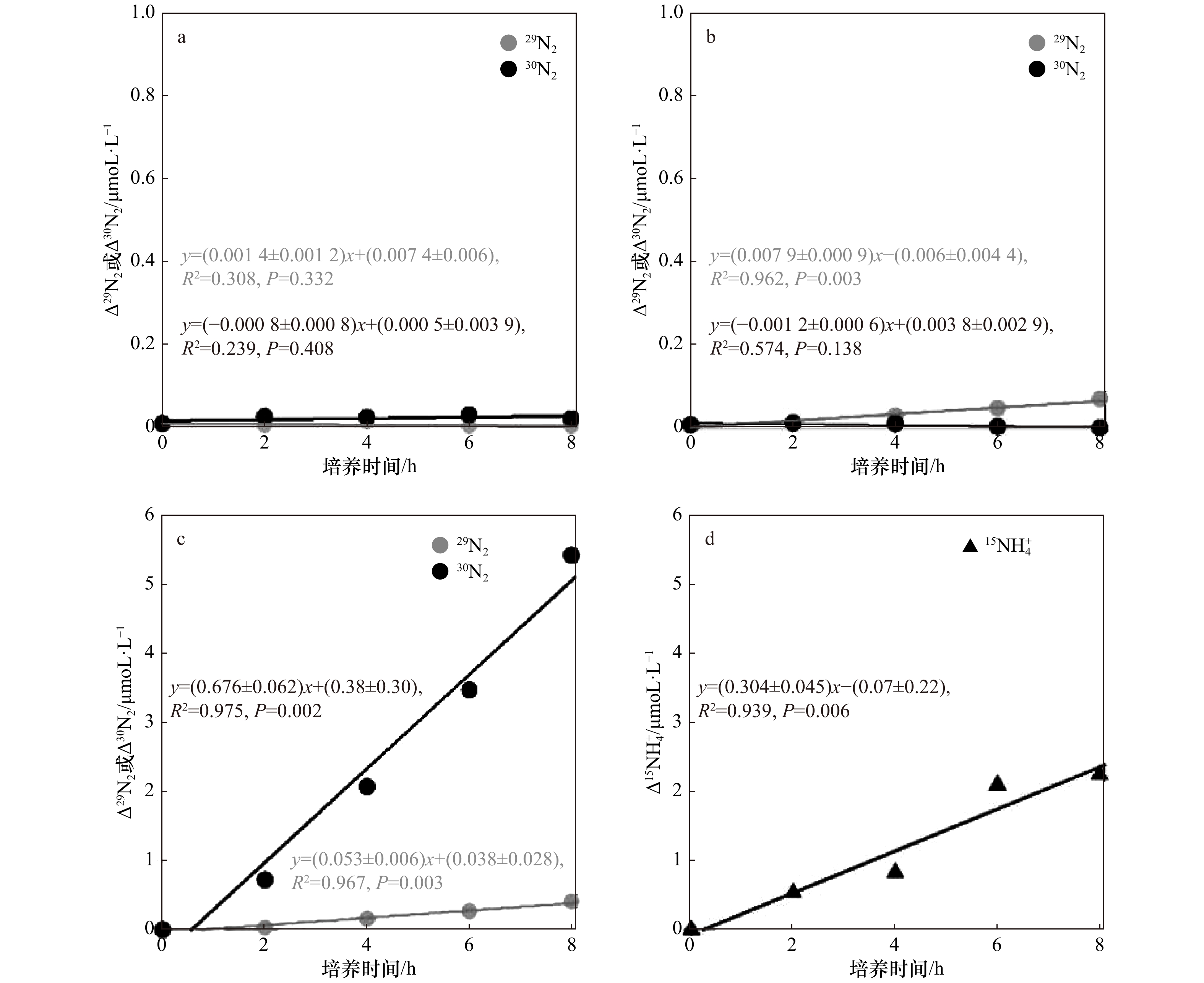
图 6 厌氧条件下石老人沙滩沉积物添加15N培养体系中29N2、30N2和
$ ^{15}{\rm{NH}}_4^ + $ 的变化a.添加$ ^{15}{\rm{NH}}_4^ + $,b.添加$ ^{15}{\rm{NH}}_4^ + $+$^{14}{\rm{NO}}_3^ - $,c. 添加$^{15}{\rm{NO}}_3^ - $,d.添加$^{15}{\rm{NO}}_3^ - $
Fig. 6 Changes of 29N2, 30N2 and
$ ^{15}{\rm{NH}}_4^ + $ in 15N culture system of sediment in Shilaoren Beach under anaerobic conditionsa. Added $ ^{15}{\rm{NH}}_4^ + $, b. added $ ^{15}{\rm{NH}}_4^ + $+$^{14}{\rm{NO}}_3^ - $, c. added $^{15}{\rm{NO}}_3^ - $, d. added $^{15}{\rm{NO}}_3^ - $
-
[1] Gruber N, Galloway J N. An earth-system perspective of the global nitrogen cycle[J]. Nature, 2008, 451(7176): 293−296. doi: 10.1038/nature06592 [2] Cai Weijun, Hu Xinping, Huang W J, et al. Acidification of subsurface coastal waters enhanced by eutrophication[J]. Nature Geoscience, 2011, 4(11): 766−770. doi: 10.1038/ngeo1297 [3] Seitzinger S P, Phillips L. Nitrogen stewardship in the Anthropocene[J]. Science, 2017, 357(6349): 350−351. doi: 10.1126/science.aao0812 [4] Breitburg D, Levin L A, Oschlies A, et al. Declining oxygen in the global ocean and coastal waters[J]. Science, 2018, 359(6371): eaam7240. doi: 10.1126/science.aam7240 [5] Seitzinger S P. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance[J]. Limnology and Oceanography, 1988, 33: 702−724. [6] Codispoti L A. An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory[J]. Biogeosciences, 2007, 4(2): 233−253. doi: 10.5194/bg-4-233-2007 [7] Devol A H. Denitrification, anammox, and N2 production in marine sediments[J]. Annual Review of Marine Science, 2015, 7: 403−423. doi: 10.1146/annurev-marine-010213-135040 [8] Gao Hang, Schreiber F, Collins G, et al. Aerobic denitrification in permeable Wadden Sea sediments[J]. The ISME Journal, 2010, 4(3): 417−426. doi: 10.1038/ismej.2009.127 [9] Marchant H K, Holtappels M, Lavik G, et al. Coupled nitrification-denitrification leads to extensive N loss in subtidal permeable sediments[J]. Limnology and Oceanography, 2016, 61(3): 1033−1048. doi: 10.1002/lno.10271 [10] Huettel M, Berg P, Kostka J E. Benthic exchange and biogeochemical cycling in permeable sediments[J]. Annual Review of Marine Science, 2014, 6: 23−51. doi: 10.1146/annurev-marine-051413-012706 [11] Sokoll S, Lavik G, Sommer S, et al. Extensive nitrogen loss from permeable sediments off North-West Africa[J]. Journal of Geophysical Research: Biogeosciences, 2016, 121(4): 1144−1157. doi: 10.1002/2015JG003298 [12] Robertson E K, Bartoli M, Brüchert V, et al. Application of the isotope pairing technique in sediments: Use, challenges, and new directions[J]. Limnology and Oceanography: Methods, 2019, 17(2): 112−136. doi: 10.1002/lom3.10303 [13] Nielsen L P. Denitrification in sediment determined from nitrogen isotope pairing[J]. FEMS Microbiology Letter, 1992, 86(4): 357−362. doi: 10.1111/j.1574-6968.1992.tb04828.x [14] Thamdrup B, Dalsgaard T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments[J]. Applied and Environmental Microbiology, 2002, 68(3): 1312−1318. doi: 10.1128/AEM.68.3.1312-1318.2002 [15] Kana T M, Darkangelo C, Hunt M D, et al. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples[J]. Analytical Chemistry, 1994, 66(23): 4166−4170. doi: 10.1021/ac00095a009 [16] 陈能汪, 吴杰忠, 段恒轶, 等. N2:Ar法直接测定水体反硝化产物溶解N2[J]. 环境科学学报, 2010, 30(12): 2479−2483.Chen Nengwang, Wu Jiezhong, Duan Hengyi, et al. N2:Ar method for direct measurement of denitrification product (dissolved N2) using membrane inlet mass spectrometry (MIMS)[J]. Acta Scientiae Circumstantiae, 2010, 30(12): 2479−2483. [17] 郑文静, 韩玉, 秦川, 等. 利用膜进样质谱连续走航测定表层海水O2/Ar比值和pCO2[J]. 海洋环境科学, 2016, 35(4): 611−617.Zheng Wenjing, Han Yu, Qin Chuan, et al. Continuous underway measurements of sea surface O2/Ar and pCO2 by membrane inlet mass spectrometry[J]. Marine Environmental Science, 2016, 35(4): 611−617. [18] An S, Gardner W S, Kana T. Simultaneous measurement of denitrification and nitrogen fixation using isotope pairing with membrane inlet mass spectrometry analysis[J]. Applied and Environmental Microbiology, 2001, 67(3): 1171−1178. doi: 10.1128/AEM.67.3.1171-1178.2001 [19] Yin Guoyu, Hou Lijun, Liu Min, et al. A novel membrane inlet mass spectrometer method to measure ${{{}^{15}}{\rm{NH}}_4^+} $ for isotope-enrichment experiments in aquatic ecosystems[J]. Environmental Science & Technology, 2014, 48(16): 9555−9562.[20] Lin Xianbiao, Liu Min, Hou Lijun, et al. Nitrogen losses in sediments of the East China Sea: spatiotemporal variations, controlling factors, and environmental implications[J]. Journal of Geophysical Research: Biogeosciences, 2017, 122(10): 2699−2715. doi: 10.1002/2017JG004036 [21] 赵永强, 夏永秋, 李博伦, 等. 利用膜进样质谱同时测定河流沉积物反硝化和厌氧氨氧化[J]. 农业环境科学学报, 2014, 33(4): 794−802. doi: 10.11654/jaes.2014.04.026Zhao Yongqiang, Xia Yongqiu, Li Bolun, et al. Simultaneous determination of denitrification and anaerobic ammonium oxidation in river sediments using membrane inlet mass spectrometry[J]. Journal of Agro-Environment Science, 2014, 33(4): 794−802. doi: 10.11654/jaes.2014.04.026 [22] Eyre B D, Rysgaard S, Dalsgaard T, et al. Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification—assumption, modifications, and implications[J]. Estuaries, 2002, 25(6): 1077−1087. doi: 10.1007/BF02692205 [23] Song G D, Liu S M, Marchant H, et al. Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment[J]. Biogeosciences, 2013, 10(11): 6851−6864. doi: 10.5194/bg-10-6851-2013 [24] Song Guodong, Liu Sumei, Zhu Zhuoyi, et al. Sediment oxygen consumption and benthic organic carbon mineralization on the continental shelves of the East China Sea and the Yellow Sea[J]. Deep Sea Research Part II: Topical Studies in Oceanography, 2016, 124: 53−63. doi: 10.1016/j.dsr2.2015.04.012 [25] Thamdrup B, Dalsgaard T. The fate of ammonium in anoxic manganese oxide-rich marine sediment[J]. Geochimica et Cosmochimica Acta, 2000, 64(24): 4157−4164. doi: 10.1016/S0016-7037(00)00496-8 [26] Sgouridis F, Stott A, Ullah S. Application of the 15N gas-flux method for measuring in situ N2 and N2O fluxes due to denitrification in natural and semi-natural terrestrial ecosystems and comparison with the acetylene inhibition technique[J]. Biogeosciences, 2016, 13(6): 1821−1835. doi: 10.5194/bg-13-1821-2016 [27] Lunstrum A, Aoki L R. Oxygen interference with membrane inlet mass spectrometry may overestimate denitrification rates calculated with the isotope pairing technique[J]. Limnology and Oceanography: Methods, 2016, 14(7): 425−431. doi: 10.1002/lom3.10101 [28] Kana T M, Weiss D L, Eyre B D, et al. Comment on “Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification” by B. D. Eyre, S. Rysgaard, T. Dalsgaard, and P. Bondo Christensen. 2002. Estuaries 25:1077–1087[J]. Estuaries, 2004, 27(1): 173−176. doi: 10.1007/BF02803571 [29] Song G D, Liu S M, Kuypers M M M, et al. Application of the isotope pairing technique in sediments where anammox, denitrification, and dissimilatory nitrate reduction to ammonium coexist[J]. Limnology and Oceanography: Methods, 2016, 14(12): 801−815. doi: 10.1002/lom3.10127 [30] Nicholls J C, Trimmer M. Widespread occurrence of the anammox reaction in estuarine sediments[J]. Aquatic Microbial Ecology, 2009, 55(2): 105−113. [31] Cook P L M, Kessler A J, Eyre B D. Does denitrification occur within porous carbonate sand grains?[J]. Biogeosciences, 2017, 14(18): 4061−4069. doi: 10.5194/bg-14-4061-2017 -



 下载:
下载: