Bacterial community structure and assembly mechanisms in Sansha Bay, Fujian
-
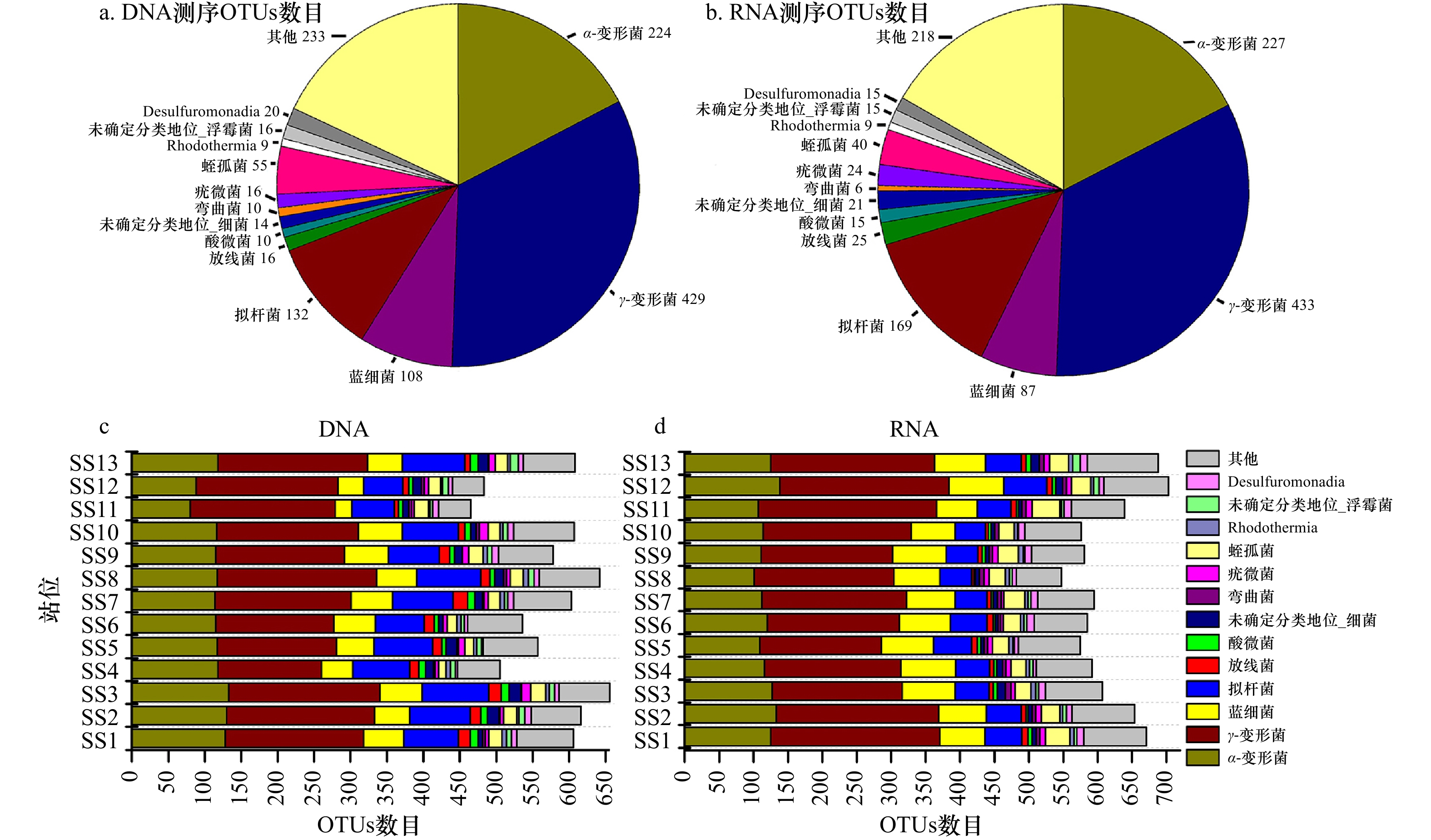
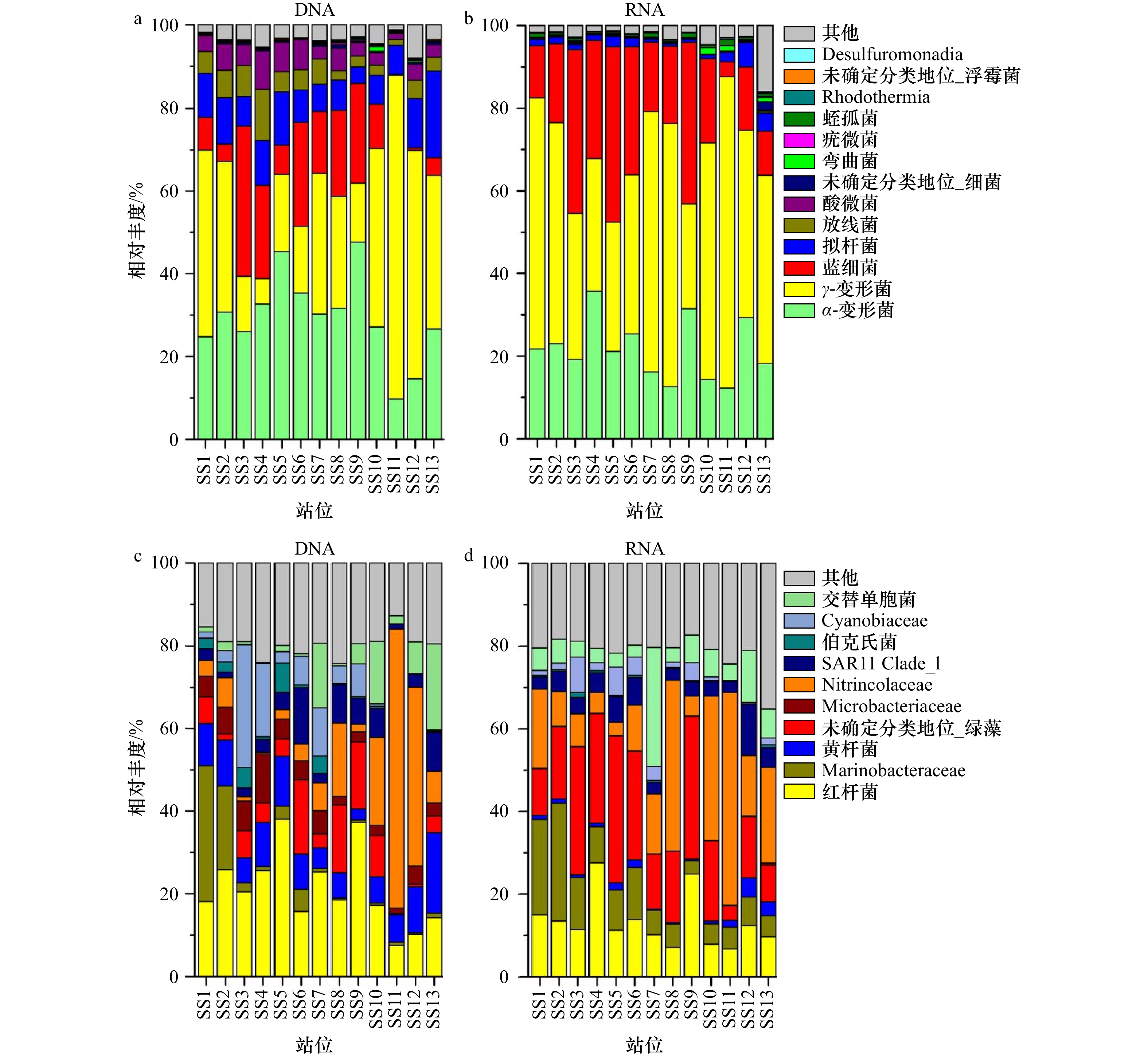
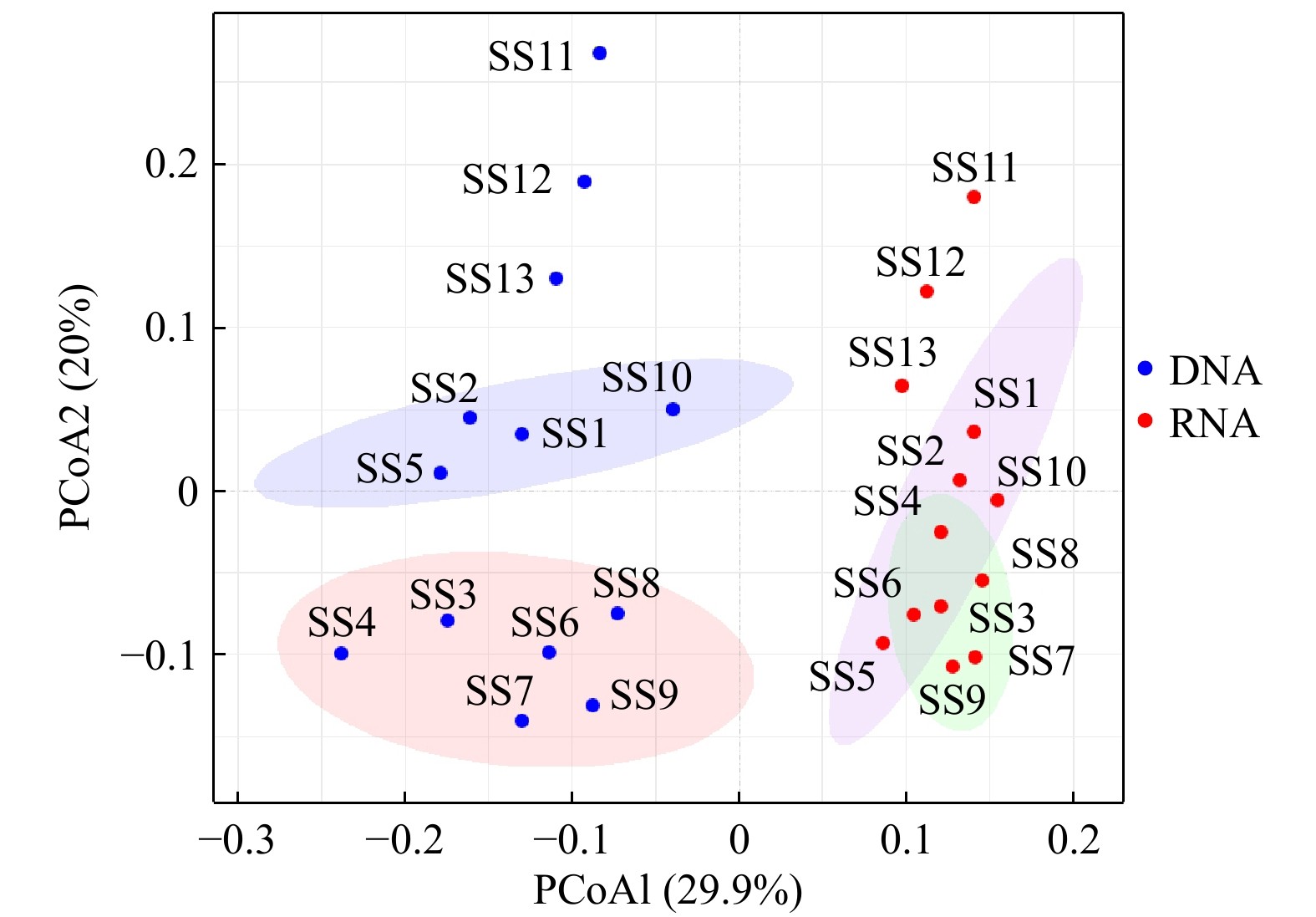
摘要: 细菌群落在水生生态系统中起着非常关键的作用。基于DNA和RNA高通量测序技术研究了福建三沙湾海域细菌的群落结构及其形成机制。结果发现:(1)三沙湾海域中共检测到细菌1 476个操作分类单元(OTUs),其中γ-变形菌、α-变形菌、蓝细菌和拟杆菌为多样性最高的类群;(2)基于DNA和RNA高通量测序技术均发现这4种类群同时也是该海域细菌群落中的优势类群,但其代谢活性处于不同的状态,主要受到盐度、总氮、亚硝氮和无机磷酸盐浓度的调控;(3)三沙湾细菌群落结构在空间尺度上的分布存在差异,表现为地理位置上越相近的海区其细菌群落结构越相似。中性模型进一步分析发现,三沙湾海域细菌群落的形成主要受到中性过程的调控。本研究结果可为深入理解福建三沙湾海域中细菌群落结构及其形成机制提供理论依据。Abstract: Bacteria play an important role in the aquatic ecosystem. In our work, bacterial community structure and assembly mechanisms were studied in Sansha Bay in Fujian Province based on DNA and RNA high-throughput sequencing. Our results revealed that: (1) A total of 1476 operational taxonomic units (OTUs) were detected, and γ-proteobacteria, α-proteobacteria, cyanobacteriia and bacteroidia were the most diverse bacterial groups. (2) γ-proteobacteria, α-proteobacteria, cyanobacteriia and bacteroidia were also the most abundant groups in Sansha Bay both in the DNA and RNA high-throughput sequencing results. The metabolic activities of these four dominant groups were different, and their metabolic activities were mainly regulated by salinity, total nitrogen, nitrite nitrogen and inorganic phosphate concentrations. (3) The bacterial community structure in Sansha Bay was different in spatial scale, and the closer the geographical location was, the more similar the bacterial community structure was. Neutral process was the main assembly mechanism affecting the construction of bacterial community in Sansha Bay. Our results were useful for the understanding the bacterial community structure and assembly mechanism in Sansha Bay, Fujian.
-
图 3 基于DNA和RNA测序技术表征的三沙湾细菌OTUs数目与温度、盐度、总氮(TN)浓度、氨氮(
${{\rm {NH}}_4^+} $ -N)浓度、硝态氮(${{\rm {NO}}_3^-} $ -N)浓度、亚硝氮(NIT)浓度、总磷(TP)浓度和正磷酸盐(SRP)浓度间的皮尔森相关性分析Fig. 3 Pearson correlations between the numbers of OTUs of bacteria and temperature, salinity, total nitrogen (TN) concentration, ammonia nitrogen (
${{\rm {NH}}_4^+} $ -N) concentration, nitrate nitrogen (${{\rm {NO}}_3^-} $ -N) concentration, nitrous nitrogen (NIT) concentration, total phosphorus (TP) concentration and soluble reactive phosphate (SRP) concentration based on the DNA and RNA sequencing data图 8 基于DNA(a)和RNA(b)表征的细菌OTUs出现频次和相对丰度间的中性模型预测图
频率高于模型预测值的OTUs显示为红色;频率较低的OTUs显示为绿色;预测范围内的OTUs显示为黑色;蓝色虚线表示模型预测的95%置信区间
Fig. 8 The neutral community model based on the relationship between bacterial OTUs occurrence and relative read abundance characterized by DNA (a) and RNA (b) approaches
OTUs with frequencies higher than those predicted by the model are displayed in red; OTUs with lower frequencies are displayed in green; OTUs within the prediction range are displayed in black; the dotted line represents the 95% confidence interval around the model prediction (blue dotted line)
表 1 各站位温度、盐度及营养盐浓度
Tab. 1 Temperature, salinity and nutrient concentrations at each sampling station
站位 温度/℃ 盐度 TN浓度/(mg·L−1) ${{\rm {NH}}_4^+} $-N浓度/(mg·L−1) $ {{\rm {NO}}_3^-} $-N浓度/(mg·L−1) NIT 浓度/(mg·L−1) TP 浓度/(mg·L−1) SRP浓度/(mg·L−1) SS1 26.7 29.1 1.165 0.306 0.393 0.089 0.111 0.073 SS2 27.1 30 1.136 0.203 0.410 0.089 0.090 0.081 SS3 26.9 21.2 1.432 0.203 0.548 0.087 0.103 0.062 SS4 27.7 22.5 1.907 0.227 0.501 0.094 0.111 0.058 SS5 27.2 27.8 1.363 0.766 0.371 0.099 0.094 0.085 SS6 28.5 23.5 1.103 0.211 0.522 0.093 0.137 0.096 SS7 26.8 17.1 1.706 0.274 0.643 0.074 0.120 0.104 SS8 27.1 22 1.218 0.243 0.541 0.087 0.103 0.096 SS9 27.2 19.6 1.515 0.258 0.558 0.082 0.107 0.092 SS10 27.4 29.9 1.304 0.227 0.366 0.089 0.098 0.104 SS11 27.8 31.1 1.492 0.227 0.387 0.110 0.145 0.138 SS12 26.8 31.3 0.546 0.227 0.399 0.104 0.090 0.134 SS13 26.2 31.3 0.401 0.243 0.311 0.096 0.047 0.107 表 2 基于DNA和RNA测序数据表征的细菌群落在采样站位的α-多样性指数
Tab. 2 Bacterial alpha diversity at sampling stations based on DNA and RNA sequencing data
站位 DNA RNA Shannon Chao1 Shannon Chao1 SS1 5.41 641.01 6.16 690.22 SS2 5.74 626.41 6.03 669.75 SS3 5.44 688.15 6.24 620.27 SS4 5.35 943.55 5.91 653.27 SS5 5.56 586.33 6.07 584.51 SS6 5.94 564.28 6.38 615.13 SS7 5.74 637.28 5.78 606.78 SS8 6.20 623.26 5.83 579.31 SS9 5.86 589.08 5.86 608.98 SS10 5.92 615.96 6.03 606.22 SS11 3.22 458.17 5.07 649.07 SS12 4.64 464.09 6.19 731.50 SS13 5.51 608.15 5.93 792.89 注:表中粗体数值为各样品中多样性指数的最高值与最低值。 表 3 α-变形菌、γ-变形菌、蓝细菌和拟杆菌OTUs分类信息
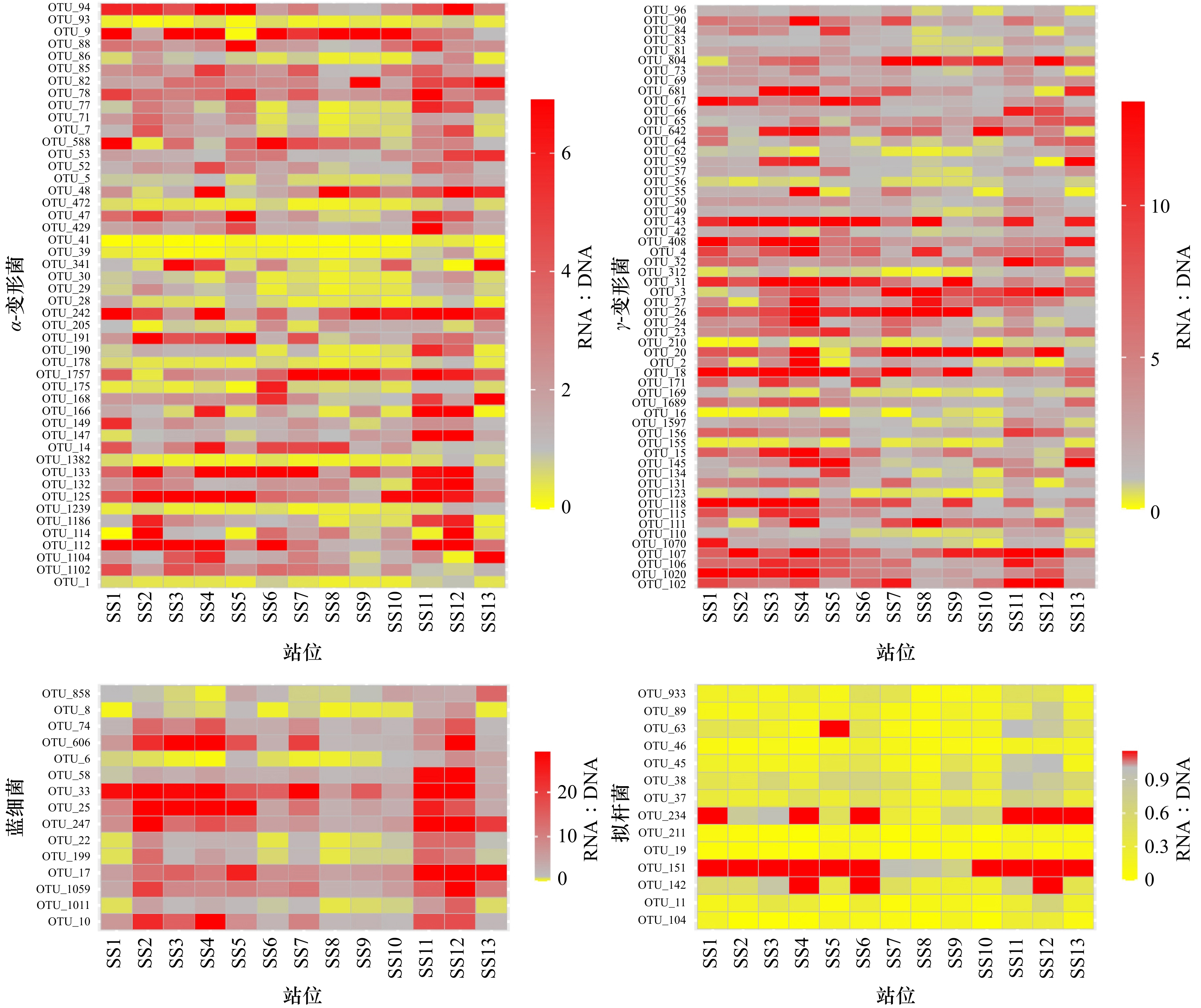
Tab. 3 The OTUs classification information of α-proteobacteria, γ-proteobacteria, Cyanobacteriia and Bacteroidia
纲 OTUs 分类信息 纲 OTUs 分类信息 Alphaproteobacteria OTU_1 Rhodobacteraceae bacterium HIMB11 Gammaproteobacteria OTU_2 Marinobacterium marisflavi OTU_5 Ascidiaceihabitans OTU_3 Marinobacter OTU_7 SAR11_clade_Ia OTU_4 Alteromonas mediterranea OTU_9 Sulfitobacter OTU_15 Neptuniibacter OTU_14 Pseudooceanicola OTU_16 Limnobacter thiooxidans OTU_28 Marivivens OTU_18 Nitrincolaceae OTU_29 SAR11_clade_III OTU_20 Ketobacter alkanivorans OTU_30 alpha proteobacterium HIMB59 OTU_23 Litoricola OTU_39 Flavimaricola OTU_24 Alteromonadaceae OTU_41 Sphingomonadaceae OTU_26 Corallomonas stylophorae OTU_47 Puniceispirillales OTU_27 Marinobacter sp. CP1 OTU_48 Hoeflea OTU_31 Marinobacterium jannaschii OTU_52 alpha proteobacterium SCGC AAA015-N04 OTU_32 Methylophilaceae OTU_53 Marivita OTU_42 SAR86_clade OTU_71 SAR11_clade OTU_43 Aestuariibacter OTU_77 SAR11_clade_III OTU_49 Nitrincolaceae OTU_78 SAR116_clade OTU_50 Pseudohongiella OTU_82 Kordiimonadales OTU_55 Glaciecola OTU_85 SAR116_clade OTU_56 OM60(NOR5)_clade OTU_86 SAR11_clade_II OTU_57 Gammaproteobacteria OTU_88 SAR116_clade OTU_59 Neptuniibacter OTU_93 Erythrobacter OTU_62 RS62 marine group OTU_94 Parvibaculaceae OTU_64 Vibrio fortis OTU_112 Alphaproteobacteria OTU_65 Polycyclovorans OTU_114 Parvularcula OTU_66 Methylophilaceae OTU_125 Magnetospiraceae OTU_67 Pseudoalteromonas phenolica OTU_132 Nisaeaceae OTU_69 Gammaproteobacteria OTU_133 AEGEAN-169_marine group OTU_73 Halioglobus OTU_147 Stappiaceae OTU_81 SAR86_clade OTU_149 NRL2 OTU_83 Luminiphilus OTU_166 Sulfitobacter OTU_84 SUP05 cluster OTU_168 Magnetospiraceae OTU_90 OM60(NOR5) clade OTU_175 Planktomarina OTU_96 SAR86 clade OTU_178 Ruegeria sp. OTU_102 Ga0077536 OTU_190 SAR11_clade_Ib OTU_106 Gammaproteobacteria OTU_191 SAR116_clade OTU_155 UBA10353 marine group OTU_205 Brevundimonas vesicularis OTU_156 Methylophilaceae OTU_242 Maricaulis maris OTU_169 EPR3968-O8a-Bc78 OTU_341 Rhodobacteraceae OTU_171 Bdellovibrionaceae OTU_429 Candidatus Puniceispirillum OTU_210 unidentified Gammaproteobacteria OTU_472 Rhodobacteraceae OTU_312 Thiotrichaceae OTU_588 Rhodobacteraceae OTU_408 Pontibacterium granulatum OTU_1102 Rhodobacteraceae OTU_642 Marinobacter salarius OTU_1104 Thalassococcus OTU_681 Neptuniibacter OTU_1186 SAR11 clade_I OTU_804 Marinobacter OTU_1239 Rhodobacteraceae OTU_1020 Pontibacterium OTU_1382 Rhodobacteraceae OTU_1070 Halieaceae OTU_1757 Limimaricola OTU_1597 SAR86_clade Cyanobacteriia OTU_6 Cyanobium PCC-6307 OTU_>1689 Neptuniibacter OTU_8 bacterium WHC4-8 OTU_11 Aureimarina OTU_10 unidentified Chloroplast OTU_19 NS5 marine group OTU_17 Virgulinella fragilis OTU_37 Cryomorphaceae OTU_22 Micromonas commoda OTU_38 NS4 marine group OTU_25 unidentified Chloroplast OTU_45 Flavobacteriaceae OTU_33 environmental clone OCS162 OTU_46 Crocinitomicaceae OTU_58 Minutocellus sp. CCMP1701 OTU_63 Cryomorphaceae OTU_74 Phalacroma mitra OTU_89 NS5 marine group OTU_199 unidentified Chloroplast OTU_104 NS4 marine group OTU_247 unidentified Chloroplast Bacteroidia OTU_142 NS9 marine group OTU_606 Guillardia theta OTU_151 NS9 marine group OTU_858 Cyanobium PCC-6307 OTU_211 NS5 marine group OTU_1011 Bdellovibrionacea OTU_234 NS9 marine group OTU_1059 unidentified Chloroplast OTU_933 Cryomorphaceae OTU_107 Nitrosococcaceae OTU_110 Nitrosomonadaceae OTU_111 Marinobacter OTU_115 bacterium BW3SW2 OTU_118 Oceanobacter kriegii OTU_123 Nitrosomonadaceae OTU_131 Amphritea OTU_134 Pseudohongiella OTU_145 Marinobacter litoralis -
[1] 中国海湾志编纂委员会. 中国海湾志(第七分册)[M]. 北京: 海洋出版社, 1994: 45-50.China Gulf Chronicles Compilation Committee. China Bay Chronicle (Volume 7)[M]. Beijing: China Ocean Press, 1994: 45−50. [2] 王萱, 刘义峰, 郭伟. 近十年三沙湾海水增养殖区环境质量状况与变化趋势评价[J]. 渔业研究, 2019, 41(6): 519−525.Wang Xuan, Liu Yifeng, Guo Wei. Evaluation of environmental quality and change trend in Sansha Bay mariculture area in recent ten years[J]. Journal of Fisheries Research, 2019, 41(6): 519−525. [3] 周进, 纪炜炜. 三都澳大型底栖动物次级生产力[J]. 海洋渔业, 2012, 34(1): 32−38. doi: 10.3969/j.issn.1004-2490.2012.01.005Zhou Jin, Ji Weiwei. Secondary productivity of macrobenthos in Sandu Bay[J]. Marine Fisheries, 2012, 34(1): 32−38. doi: 10.3969/j.issn.1004-2490.2012.01.005 [4] 黄伟强, 纪炜炜, 付婧, 等. 三沙湾大黄鱼网箱养殖衍生有机物的沉降特征[J]. 中国水产科学, 2020, 27(6): 709−719.Huang Weiqiang, Ji Weiwei, Fu Jing, et al. Sedimentation characteristics of aquaculture-derived organic matter from a large yellow croaker (Larimichthys crocea) cage farm in Sansha Bay[J]. Journal of Fishery Sciences of China, 2020, 27(6): 709−719. [5] Freimann R, Bürgmann H, Findlay S E, et al. Bacterial structures and ecosystem functions in glaciated floodplains: contemporary states and potential future shifts[J]. The ISME Journal, 2013, 7(12): 2361−2373. doi: 10.1038/ismej.2013.114 [6] 唐娅菲, 王金辉, 程宏, 等. 三沙湾春季浮游植物群落结构及其与环境因子的关系[J]. 上海海洋大学学报, 2018, 27(4): 522−530. doi: 10.12024/jsou.20170802123Tang Yafei, Wang Jinhui, Cheng Hong, et al. Community structure of phytoplankton and its relationship with environmental factors of Sansha Bay in spring[J]. Journal of Shanghai Ocean University, 2018, 27(4): 522−530. doi: 10.12024/jsou.20170802123 [7] 徐佳奕, 徐兆礼. 三沙湾浮游动物生态类群演替特征[J]. 生态学报, 2013, 33(5): 1413−1424. doi: 10.5846/stxb201207241050Xu Jiayi, Xu Zhaoli. Seasonal succession of zooplankton in Sansha Bay, Fujian[J]. Acta Ecologica Sinica, 2013, 33(5): 1413−1424. doi: 10.5846/stxb201207241050 [8] Wang Feipeng, Huang Bangqin, Xie Yuyuan, et al. Diversity, composition, and activities of nano- and pico-eukaryotes in the northern South China Sea with influences of Kuroshio intrusion[J]. Frontiers in Marine Science, 2021, 8: 658233. doi: 10.3389/fmars.2021.658233 [9] Massana R, Gobet A, Audic S, et al. Marine protist diversity in European coastal waters and sediments as revealed by high-throughput sequencing[J]. Environmental Microbiology, 2015, 17(10): 4035−4049. doi: 10.1111/1462-2920.12955 [10] De Vargas C, Audic S, Henry N, et al. Eukaryotic plankton diversity in the Sunlit ocean[J]. Science, 2015, 348(6237): 1261605. doi: 10.1126/science.1261605 [11] Not F, del Campo J, Balagué V, et al. New insights into the diversity of marine picoeukaryotes[J]. PLoS One, 2009, 4(9): e7143. doi: 10.1371/journal.pone.0007143 [12] Hu S K, Campbell V, Connell P, et al. Protistan diversity and activity inferred from RNA and DNA at a coastal ocean site in the eastern North Pacific[J]. FEMS Microbiology Ecology, 2016, 92(4): fiw050. [13] Wang Feipeng, Xie Yuyuan, Wu Wenxue, et al. Picoeukaryotic diversity and activity in the northwestern Pacific Ocean based on rDNA and rRNA high-throughput sequencing[J]. Frontiers in Microbiology, 2019, 9: 3259. doi: 10.3389/fmicb.2018.03259 [14] Xu Dapeng, Li Ran, Hu Chen, et al. Microbial eukaryote diversity and activity in the water column of the South China Sea based on DNA and RNA high throughput sequencing[J]. Frontiers in Microbiology, 2017, 8: 1121. doi: 10.3389/fmicb.2017.01121 [15] Wu Wenxue, Liu Hongbin. Disentangling protist communities identified from DNA and RNA surveys in the Pearl River-South China Sea continuum during the wet and dry seasons[J]. Molecular Ecology, 2018, 27(22): 4627−4640. doi: 10.1111/mec.14867 [16] Blazewicz S J, Barnard R L, Daly R A, et al. Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses[J]. The ISME Journal, 2013, 7(11): 2061−2068. doi: 10.1038/ismej.2013.102 [17] 国家环境保护总局, 《水和废水监测分析方法》编委会. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002.State Environmental Protection Administration of China, Editorial Committee of Water and Wastewater Monitoring and Analysis Methods. Water and Wastewater Monitoring and Analysis Methods[M]. 4th Ed. Beijing: China Environmental Science Press, 2002. [18] He Shuiqing, Li Dan, Wang Feipeng, et al. Parental exposure to sulfamethazine and nanoplastics alters the gut microbial communities in the offspring of marine madaka (Oryzias melastigma)[J]. Journal of Hazardous Materials, 2022, 423: 127003. [19] Magoč T, Salzberg S L. FLASH: fast length adjustment of short reads to improve genome assemblies[J]. Bioinformatics, 2011, 27(21): 2957−2963. doi: 10.1093/bioinformatics/btr507 [20] Caporaso J G, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data[J]. Nature Methods, 2010, 7(5): 335−336. doi: 10.1038/nmeth.f.303 [21] Rognes T, Flouri T, Nichols B, et al. VSEARCH: a versatile open source tool for metagenomics[J]. PeerJ, 2016, 4: e2584. doi: 10.7717/peerj.2584 [22] Edgar R C. UPARSE: highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 2013, 10(10): 996−998. doi: 10.1038/nmeth.2604 [23] Wang Qiong, Garrity G M, Tiedje J M, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy[J]. Applied and Environmental Microbiology, 2007, 73(16): 5261−5267. doi: 10.1128/AEM.00062-07 [24] Dixon P. VEGAN, a package of R functions for community ecology[J]. Journal of Vegetation Science, 2003, 14(6): 927−930. doi: 10.1111/j.1654-1103.2003.tb02228.x [25] Esposti M D. Bioenergetic evolution in proteobacteria and mitochondria[J]. Genome Biology and Evolution, 2014, 6(12): 3238−3251. doi: 10.1093/gbe/evu257 [26] Choi D H, Jang G II, Lapidus A, et al. Draft genome sequence of Marinobacterium rhizophilum CL-YJ9T (DSM 18822T), isolated from the rhizosphere of the coastal tidal-flat plant Suaeda japonica[J]. Standards in Genomic Sciences, 2017, 12: 65. doi: 10.1186/s40793-017-0275-x [27] Liu Shuting, Wawrik B, Liu Zhanfei. Different bacterial communities involved in peptide decomposition between Normoxic and hypoxic coastal waters[J]. Frontiers in Microbiology, 2017, 8: 353. [28] Barbeau K, Zhang Guangping, Live D H, et al. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus[J]. Journal of the American Chemical Society, 2002, 124(3): 378−379. doi: 10.1021/ja0119088 [29] DIéguez A L, Romalde J L. Draft genome sequences of Neptuniibacter sp. strains LFT 1.8 and ATR 1.1[J]. Genome Announcements, 2017, 5(5): e01541−16. [30] Tremblay J, Yergeau E, Fortin N, et al. Chemical dispersants enhance the activity of oil- and gas condensate-degrading marine bacteria[J]. The ISME Journal, 2017, 11(12): 2793−2808. doi: 10.1038/ismej.2017.129 [31] Zheng Li, Cui Zhisong, Xu Luyan, et al. Draft genome sequence of Rhodobacteraceae strain PD-2, an algicidal bacterium with a quorum-sensing system, isolated from the marine microalga Prorocentrum donghaiense[J]. Genome Announcements, 2015, 3(1): e01549−14. [32] Zhang Mengyu, Pan Luqing, Huang Fei, et al. Metagenomic analysis of composition, function and cycling processes of microbial community in water, sediment and effluent of Litopenaeus vannamei farming environments under different culture modes[J]. Aquaculture, 2019, 506: 280−293. doi: 10.1016/j.aquaculture.2019.03.038 [33] Flombaum P, Gallegos J L, Gordillo R A, et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(24): 9824−9829. doi: 10.1073/pnas.1307701110 [34] Barbeyron T, Thomas F, Barbe V, et al. Habitat and taxon as driving forces of carbohydrate catabolism in marine heterotrophic bacteria: example of the model algae-associated bacterium Zobellia galactanivorans DsijT[J]. Environmental Microbiology, 2016, 18(12): 4610−4627. doi: 10.1111/1462-2920.13584 [35] 王飞鹏, 黄亚玲, 张瑞瑞, 等. 不同曝气方式对人工湿地细菌多样性、代谢活性及功能的影响[J]. 环境科学, 2022, 43(4): 2007−2017. doi: 10.13227/j.hjkx.202107135Wang Feipeng, Huang Yaling, Zhang Ruirui, et al. Effects of different aeration treatments on bacterial diversity, metabolic activity, and function in constructed wetlands[J]. Environmental Science, 2022, 43(4): 2007−2017. doi: 10.13227/j.hjkx.202107135 [36] Xu Zhimeng, Cheung S, Endo H, et al. Disentangling the ecological processes shaping the latitudinal pattern of phytoplankton communities in the Pacific Ocean[J]. mSystems, 2022, 7(1): e0120321. doi: 10.1128/msystems.01203-21 [37] Kong Jie, Wang Lei, Lin Cai, et al. Contrasting community assembly mechanisms underlie similar biogeographic patterns of surface microbiota in the tropical north Pacific Ocean[J]. Microbiology Spectrum, 2022, 10(1): e0079821. doi: 10.1128/spectrum.00798-21 [38] Vellend M. The Theory of Ecological Communities (MPB-57)[M]. Princeton: Princeton University Press, 2016. [39] Chave J. Neutral theory and community ecology[J]. Ecology Letters, 2004, 7(3): 241−253. doi: 10.1111/j.1461-0248.2003.00566.x [40] Chen Weidong, Ren Kexin, Isabwe A, et al. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons[J]. Microbiome, 2019, 7(1): 138. doi: 10.1186/s40168-019-0749-8 [41] Burns A R, Stephens W Z, Stagaman K, et al. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development[J]. The ISME Journal, 2016, 10(3): 655−664. doi: 10.1038/ismej.2015.142 -





 下载:
下载: