Determination of Cu and Zn isotopes in sediments by multi-collector inductively coupled plasma mass spectrometer
-
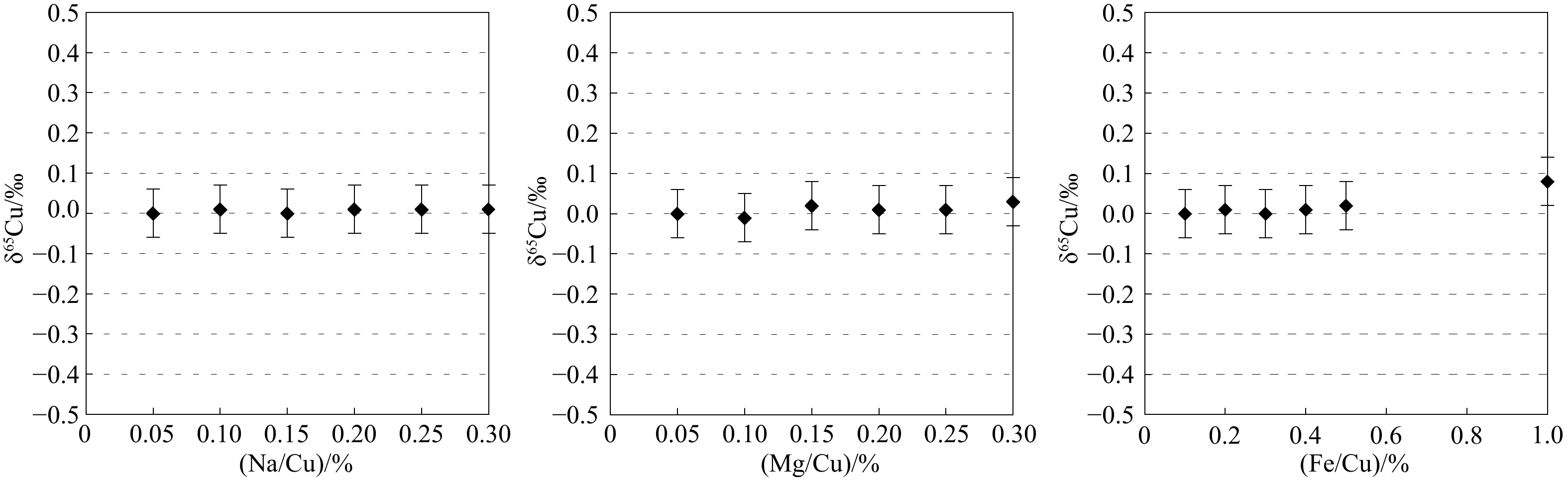
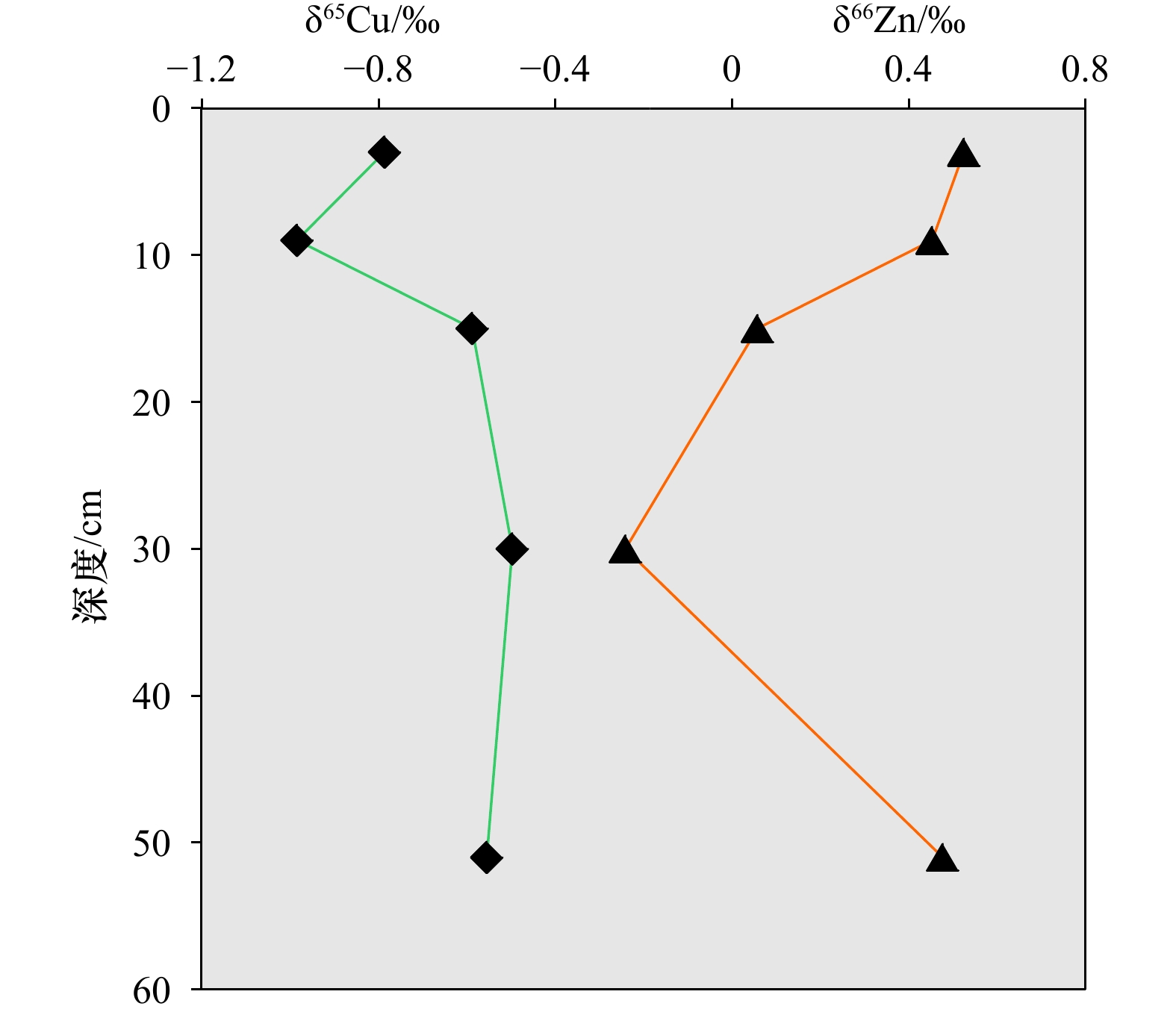
摘要: 本文介绍了海洋沉积物中Cu和Zn同位素的化学预处理及测定方法,报道了冲绳海槽20件表层沉积物和5件柱状沉积物样品的Cu和Zn同位素组成。采用大孔径阴离子交换树脂AG MP-1M,分别以8.2 mol/L HCl+0.01%HF+0.001%H2O2、2 mol/L HCl+0.001%H2O2和0.5 mol/L HNO3作为淋洗液,能有效分离海洋沉积物中的基质元素和Cu、Zn元素,且Cu和Zn的回收率均接近100%。以内标法和标准−样品−标准法联合校正多接收电感耦合等离子体质谱仪的质量歧视,δ65Cu和δ66Zn的分析精度分别为0.11‰和0.09‰(2SD)。冲绳海槽表层沉积物δ66Zn分布范围为0.07‰~0.67‰,δ66Zn平均值为0.31‰±0.32‰(2SD);δ65Cu的分布范围为−2.26‰~−0.52‰,δ65Cu平均值为−1.21‰±0.55‰(2SD)。表层沉积物δ66Zn和δ65Cu分布范围较大,柱状沉积物样品δ66Zn和δ65Cu值随深度存在较显著变化。
-
关键词:
- Cu同位素 /
- Zn同位素 /
- 多接收电感耦合等离子体质谱 /
- 海洋沉积物 /
- 冲绳海槽
Abstract: We presented an optimized and purification procedure as well as an analytical method for Cu and Zn isotopes measurement in marine sediments. We reported Cu and Zn isotope of 5 samples in a sediment cores and 20 surface sediment samples in the northern and southern Okinawa Trough. Anion exchange resin (AG MP-1M) was applied to separate matrix elements of sediment samples from Cu, Fe and Zn using 8.2 mol/L HCl+0.01% HF+0.001% H2O2, 2 mol/L HCl+0.001% H2O2 and 0.5 mol/L HNO3 as eluents. The recoveries of Cu and Zn were both close to 100%. Cu and Zn isotopes were measured on a Nu Plasma multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS). Instrumental mass bias was corrected using a combination of sample-standard bracketing and internal spiking. The long-term reproducibilities were 0.11‰ (2SD) for Cu isotopes and 0.09‰ (2SD) for Zn isotopes. The δ66Zn of surface sediments varies from 0.07‰ to 0.67‰, with an average of 0.31‰ ± 0.32‰ (2SD); the δ65Cu of surface sediments ranges from −2.26‰ to −0.52‰, with an average of −1.21‰ ± 0.55‰ (2SD). The results show that Cu and Zn isotopes of surface sediments in the Okinawa Trough varies widely, meanwhile, Cu and Zn isotopes in sediment core varies with depth. -
表 1 化学分离流程
Tab. 1 Chemical separation procedure
色谱柱 AG-MP-1M 树脂 清洗树脂 5 mL H2O 平衡树脂 4 mL 8.2 mol/L HCl 上样 1 mL 8.2 mol/L HCl 淋洗基质元素 5 mL 8.2 mol/L HCl + 0.001% H2O2 淋洗并接收Cu 20 mL 8.2 mol/L HCl + 0.01% HF + 0.001% H2O2 淋洗并接收Fe 15 mL 2 mol/L HCl + 0.001% H2O2 淋洗基质元素 2 mL 0.5 mol/L HNO3 淋洗并接收Zn 7 mL 0.5 mol/L HNO3 表 2 沉积物Cu、Zn回收率
Tab. 2 Recovery of Cu and Zn in sediments
样品 Zn含量/(μg·g−1)
(过柱前)Zn含量/(μg·g−1)
(过柱后)Zn回收率/% Cu含量/(μg·g−1)
(过柱前)Cu含量/(μg·g−1)
(过柱后)Cu回收率/% S1 146.13 145.98 99.9 41.72 41.39 99.2 S2 103.49 101.94 98.5 31.71 31.61 99.7 S3 99.38 98.78 99.4 25.65 25.52 99.5 S4 156.90 153.45 97.8 43.98 43.32 98.5 S5 485.50 483.07 99.5 205.04 203.60 99.3 S6 88.14 86.99 98.7 22.81 22.72 99.6 S7 95.85 95.47 99.6 22.52 22.25 98.8 S8 95.28 95.09 99.8 32.24 32.05 99.4 S9 100.36 99.26 98.9 26.97 26.40 97.9 S10 92.60 92.14 99.5 25.16 24.83 98.7 GBW07333 114.15 113.92 99.8 29.25 29.84 102 BHVO-2 101.56 100.14 98.6 126.62 126.24 99.7 BCR-2 126.36 125.73 99.5 18.96 18.92 99.8 注:样品回收率重复次数n=3,标准物质GBW07333重复次数n=5,BHVO-2重复次数为n=4,BCR-2重复次数n=3,ICP-MS测定Cu、Zn含量的不确定度为±8%。 表 3 冲绳海槽表层沉积物Cu和Zn同位素组成
Tab. 3 Cu and Zn isotopic compositions of the surface sediments in Okinawa Trough
样品 δ66ZnJMC-Lyon/‰ 2SD/‰ δ65CuNIST976/‰ 2SD/‰ Y7-3-4 0.57 0.06 –0.70 0.06 Y7-9-10 0.51 0.08 –0.94 0.04 Y7-9-10* 0.47 0.06 –0.99 0.06 Y7-15-16 0.18 0.07 –0.46 0.05 Y7-30-31 –0.04 0.07 –0.35 0.05 Y7-51-52 0.53 0.05 –0.42 0.04 S1 0.34 0.06 –0.95 0.06 S2 0.10 0.04 –1.06 0.05 S3 0.33 0.06 –0.85 0.07 S4 0.07 0.04 –1.57 0.04 S5 0.43 0.08 –1.44 0.05 S6 0.32 0.07 –1.14 0.06 S7 0.27 0.06 –0.63 0.04 S8 0.47 0.06 –0.59 0.07 S9 0.13 0.07 –0.64 0.05 S9* 0.16 0.07 –0.60 0.06 S10 0.29 0.06 –0.73 0.07 S11 0.18 0.08 –0.87 0.05 S12 0.39 0.09 –0.93 0.05 S13 0.24 0.07 –2.26 0.05 S14 0.44 0.06 –1.86 0.05 S15 0.20 0.05 –2.14 0.09 S16 0.14 0.08 –1.94 0.04 S17 0.56 0.06 –1.69 0.06 S18 0.39 0.09 –1.23 0.07 S19 0.67 0.08 –0.52 0.06 S20 0.48 0.07 –1.70 0.04 标准物质a BHVO-2 0.32 0.06 0.11 0.03 BHVO-2报道值 0.29±0.09 0.12±0.02 BCR-2 0.25 0.07 0.15 0.04 BCR-2报道值 0.33±0.09 0.17±0.05 注:a 标准物质的Cu和Zn同位素做了多次重复测量,BHVO-2重复次数n=4,BCR-2重复次数n=3;*表示重复样。 -
[1] Morel F M M, Price N M. The biogeochemical cycles of trace metals in the oceans[J]. Science, 2003, 300(5621): 944−947. doi: 10.1126/science.1083545 [2] John S G, Kunzmann M, Townsend E J, et al. Zinc and cadmium stable isotopes in the geological record: A case study from the post-snowball Earth Nuccaleena cap dolostone[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 466: 202−208. doi: 10.1016/j.palaeo.2016.11.003 [3] Lü Yiwen, Liu Sheng’ao, Wu Huaichun, et al. Zn-Sr isotope records of the Ediacaran Doushantuo formation in South China: Diagenesis assessment and implications[J]. Geochimica et Cosmochimica Acta, 2018, 239: 330−345. doi: 10.1016/j.gca.2018.08.003 [4] Navarrete J U, Borrok D M, Viveros M, et al. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria[J]. Geochimica et Cosmochimica Acta, 2011, 75(3): 784−799. doi: 10.1016/j.gca.2010.11.011 [5] John S G, Geis R W, Saito M A, et al. Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the marine diatom Thalassiosira oceanica[J]. Limnology and Oceanography, 2007, 52(6): 2710−2714. doi: 10.4319/lo.2007.52.6.2710 [6] Fru E C, Rodríguez N P, Partin C A, et al. Cu isotopes in marine black shales record the Great Oxidation Event[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(18): 4941−4946. doi: 10.1073/pnas.1523544113 [7] Liu Pingping, Teng Fangzhen, Dick H J B, et al. Magnesium isotopic composition of the oceanic mantle and oceanic Mg cycling[J]. Geochimica et Cosmochimica Acta, 2017, 206: 151−165. doi: 10.1016/j.gca.2017.02.016 [8] Sweere T C, Dickson A J, Jenkyns H C, et al. Isotopic evidence for changes in the zinc cycle during oceanic anoxic event 2 (Late Cretaceous)[J]. Geology, 2018, 46(5): 463−466. doi: 10.1130/G40226.1 [9] Wang Xun, Liu Sheng’ao, Wang Zhengrong, et al. Zinc and strontium isotope evidence for climate cooling and constraints on the Frasnian-Famennian (~372 Ma) mass extinction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2018, 498: 68−82. doi: 10.1016/j.palaeo.2018.03.002 [10] Fujii T, Moynier F, Dauphas N, et al. Theoretical and experimental investigation of nickel isotopic fractionation in species relevant to modern and ancient oceans[J]. Geochimica et Cosmochimica Acta, 2011, 75(2): 469−482. doi: 10.1016/j.gca.2010.11.003 [11] Anbar A D, Rouxel O. Metal stable isotopes in paleoceanography[J]. Annual Review of Earth and Planetary Sciences, 2007, 35: 717−746. doi: 10.1146/annurev.earth.34.031405.125029 [12] Little S H, Vance D, McManus J, et al. Copper isotope signatures in modern marine sediments[J]. Geochimica et Cosmochimica Acta, 2017, 212: 253−273. doi: 10.1016/j.gca.2017.06.019 [13] Vance D, Archer C, Bermin J, et al. The copper isotope geochemistry of rivers and the oceans[J]. Earth and Planetary Science Letters, 2008, 274(1/2): 204−213. [14] Thompson C M, Ellwood M J, Wille M. A solvent extraction technique for the isotopic measurement of dissolved copper in seawater[J]. Analytica Chimica Acta, 2013, 775: 106−113. doi: 10.1016/j.aca.2013.03.020 [15] Boyle E A, John S, Abouchami W, et al. GEOTRACES IC1 (BATS) contamination-prone trace element isotopes Cd, Fe, Pb, Zn, Cu, and Mo intercalibration[J]. Limnology and Oceanography Methods, 2012, 10(9): 653−665. doi: 10.4319/lom.2012.10.653 [16] Maréchal C N, Nicolas E, Douchet C, et al. Abundance of zinc isotopes as a marine biogeochemical tracer[J]. Geochemistry, Geophysics, Geosystems, 2000, 1(5): 1015. [17] Albarède F, Telouk P, Lamboux A, et al. Isotopic evidence of unaccounted for Fe and Cu erythropoietic pathways[J]. Metallomics, 2011, 3(9): 926−933. doi: 10.1039/c1mt00025j [18] Little S H, Vance D, Walker-Brown C, et al. The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments[J]. Geochimica et Cosmochimica Acta, 2014, 125: 673−693. doi: 10.1016/j.gca.2013.07.046 [19] Othman D B, Luck J M, Tchalikian A, et al. Cu-Zn isotope systematics in terrestrial basalts[J]. Geophysical Research Abstracts, 2003, 5: 09669. [20] Albarède F, Beard B. Analytical methods for non-traditional isotopes[J]. Reviews in Mineralogy and Geochemistry, 2004, 55(1): 113−152. doi: 10.2138/gsrmg.55.1.113 [21] Mason T F D, Weiss D J, Horstwood M, et al. High-precision Cu and Zn isotope analysis by plasma source mass spectrometry part 1. Spectral interferences and their correction[J]. Journal of Analytical Atomic Spectrometry, 2004, 19(2): 209−217. doi: 10.1039/b306958c [22] Mason T F D, Weiss D J, Horstwood M, et al. High-precision Cu and Zn isotope analysis by plasma source mass spectrometry part 2. Correcting for mass discrimination effects[J]. Journal of Analytical Atomic Spectrometry, 2004, 19(2): 218−226. doi: 10.1039/b306953b [23] Maréchal C N, Télouk P, Albarède F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry[J]. Chemical Geology, 1999, 156(1/4): 251−273. [24] Archer C, Vance D. Mass discrimination correction in multiple-collector plasma source mass spectrometry: An example using Cu and Zn isotopes[J]. Journal of Analytical Atomic Spectrometry, 2004, 19(5): 656−665. doi: 10.1039/b315853e [25] 侯可军, 李延河, 田有荣, 等. MC-ICP-MS高精度Cu、Zn同位素测试技术[J]. 矿床地质, 2008, 27(6): 774−781. doi: 10.3969/j.issn.0258-7106.2008.06.010Hou Kejun, Li Yanhe, Tian Yourong, et al. High precision Cu, Zn isotope measurements by multi-collector ICP-MS[J]. Mineral Deposits, 2008, 27(6): 774−781. doi: 10.3969/j.issn.0258-7106.2008.06.010 [26] Luck J M, Othman D B, Albarède F. Zn and Cu isotopic variations in chondrites and iron meteorites: Early solar nebula reservoirs and parent-body processes[J]. Geochimica et Cosmochimica Acta, 2005, 69(22): 5351−5363. doi: 10.1016/j.gca.2005.06.018 [27] Chapman J B, Mason T F D, Weiss D J, et al. Chemical separation and isotopic variations of Cu and Zn from five geological reference materials[J]. Geostandards and Geoanalytical Research, 2006, 30(1): 5−16. doi: 10.1111/j.1751-908X.2006.tb00907.x [28] John S G, Rouxel O J, Craddock P R, et al. Zinc stable isotopes in seafloor hydrothermal vent fluids and chimneys[J]. Earth and Planetary Science Letters, 2008, 269(1/2): 17−28. [29] Dong Shuofei, Weiss D J, Strekopytov S, et al. Stable isotope ratio measurements of Cu and Zn in mineral dust (bulk and size fractions) from the Taklimakan Desert and the Sahel and in aerosols from the eastern tropical North Atlantic Ocean[J]. Talanta, 2013, 114: 103−109. doi: 10.1016/j.talanta.2013.03.062 [30] Zhu Zhiyong, Jiang Shaoyong, Yang Tao, et al. Improvements in Cu-Zn isotope analysis with MC-ICP-MS: A revisit of chemical purification, mass spectrometry measurement and mechanism of Cu/Zn mass bias decoupling effect[J]. International Journal of Mass Spectrometry, 2015, 393: 34−40. doi: 10.1016/j.ijms.2015.10.009 [31] Araújo D F, Boaventura G R, Machado W, et al. Tracing of anthropogenic zinc sources in coastal environments using stable isotope composition[J]. Chemical Geology, 2017, 449: 226−235. doi: 10.1016/j.chemgeo.2016.12.004 [32] Balistrieri L S, Borrok D M, Wanty R B, et al. Fractionation of Cu and Zn isotopes during adsorption onto amorphous Fe(III) oxyhydroxide: Experimental mixing of acid rock drainage and ambient river water[J]. Geochimica et Cosmochimica Acta, 2008, 72(2): 311−328. doi: 10.1016/j.gca.2007.11.013 [33] Moeller K, Schoenberg R, Pedersen R B, et al. Calibration of the new certified reference materials ERM-AE633 and ERM-AE647 for copper and IRMM-3702 for zinc isotope amount ratio determinations[J]. Geostandards and Geoanalytical Research, 2012, 36(2): 177−199. doi: 10.1111/j.1751-908X.2011.00153.x [34] 李世珍, 朱祥坤, 唐索寒, 等. 多接收器等离子体质谱法Zn同位素比值的高精度测定[J]. 岩石矿物学杂志, 2008, 27(4): 273−278. doi: 10.3969/j.issn.1000-6524.2008.04.002Li Shizhen, Zhu Xiangkun, Tang Suohan, et al. The application of MC-ICP-MS to high-precision measurement of Zn isotope ratios[J]. Acta Petrologica et Mineralogica, 2008, 27(4): 273−278. doi: 10.3969/j.issn.1000-6524.2008.04.002 [35] 唐索寒, 朱祥坤, 蔡俊军, 等. 用于多接收器等离子体质谱铜铁锌同位素测定的离子交换分离方法[J]. 岩矿测试, 2006, 25(1): 5−8. doi: 10.3969/j.issn.0254-5357.2006.01.002Tang Suohan, Zhu Xiangkun, Cai Junjun, et al. Chromatographic separation of Cu, Fe and Zn using AG MP-1 anion exchange resin for isotope determination by MC-ICPMS[J]. Rock and Mineral Analysis, 2006, 25(1): 5−8. doi: 10.3969/j.issn.0254-5357.2006.01.002 [36] Gao Jingjing, Liu Jihua, Li Xianguo, et al. The determination of 52 elements in marine geological samples by an inductively coupled plasma optical emission spectrometry and an inductively coupled plasma mass spectrometry with a high-pressure closed digestion method[J]. Acta Oceanologica Sinica, 2017, 36(1): 109−117. doi: 10.1007/s13131-017-0991-5 [37] Bermin J, Vance D, Archer C, et al. The determination of the isotopic composition of Cu and Zn in seawater[J]. Chemical Geology, 2006, 226(3/4): 280−297. [38] Peel K, Weiss D, Chapman J, et al. A simple combined sample-standard bracketing and inter-element correction procedure for accurate mass bias correction and precise Zn and Cu isotope ratio measurements[J]. Journal of Analytical Atomic Spectrometry, 2008, 23(1): 103−110. doi: 10.1039/B710977F [39] Souto-Oliveira C E, Babinski M, Araújo D F, et al. Multi-isotope approach of Pb, Cu and Zn in urban aerosols and anthropogenic sources improves tracing of the atmospheric pollutant sources in megacities[J]. Atmospheric Environment, 2019, 198: 427−437. doi: 10.1016/j.atmosenv.2018.11.007 [40] Zhu X K, O’Nions R K, Guo Y, et al. Determination of natural Cu-isotope variation by plasma-source mass spectrometry: Implications for use as geochemical tracers[J]. Chemical Geology, 2000, 163(1/4): 139−149. [41] Araújo D F, Ponzevera E, Briant N, et al. Assessment of the metal contamination evolution in the Loire estuary using Cu and Zn stable isotopes and geochemical data in sediments[J]. Marine Pollution Bulletin, 2019, 143: 12−23. doi: 10.1016/j.marpolbul.2019.04.034 [42] Kříbek B, Míková J, Knésl I, et al. Uptake of trace elements and isotope fractionation of Cu and Zn by birch (Betula pendula) growing on mineralized coal waste pile[J]. Applied Geochemistry, 2020, 122: 104741. doi: 10.1016/j.apgeochem.2020.104741 [43] Liang Lili, Liu Congqiang, Zhu Xiangkun, et al. Zinc isotope characteristics in the biogeochemical cycle as revealed by analysis of suspended particulate matter (SPM) in Aha Lake and Hongfeng Lake, Guizhou, China[J]. Journal of Earth Science, 2020, 31(1): 126−140. doi: 10.1007/s12583-017-0957-8 [44] Moynier F, Vance D, Fujii T, et al. The isotope geochemistry of Zinc and Copper[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 543−600. doi: 10.2138/rmg.2017.82.13 [45] 朱爱美, 石学法, 邹建军, 等. 88 ka以来冲绳海槽北部古环境演化: 来自元素地球化学的证据[J]. 海洋学报, 2015, 37(6): 58−69.Zhu Aimei, Shi Xuefa, Zou Jianjun, et al. Paleoenvironment changes in the northern Okinawa Trough since 88 ka: Evidences from element geochemistry[J]. Haiyang Xuebao, 2015, 37(6): 58−69. [46] 赵德博, 万世明. 冲绳海槽沉积物物源示踪研究进展[J]. 海洋地质前沿, 2015, 31(2): 32−41.Zhao Debo, Wan Shiming. Research progress of tracing sediment sources in Okinawa Trough[J]. Marine Geology Frontiers, 2015, 31(2): 32−41. [47] Zheng Zhuo, Yang Shixiong, Deng Yun, et al. Pollen record of the past 60 ka BP in the Middle Okinawa Trough: Terrestrial provenance and reconstruction of the paleoenvironment[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2011, 307(1/4): 285−300. [48] Xu Hongyan, Chang Fengming, Luo Yunli, et al. Palaeoenvironmental changes from pollen record in deep sea core PC-1 from northern Okinawa Trough, East China Sea during the past 24 ka[J]. Chinese Science Bulletin, 2009, 54(20): 3739−3748. doi: 10.1007/s11434-009-0227-y [49] Dou Yanguang, Yang Shouye, Liu Zhenxia, et al. Provenance discrimination of siliciclastic sediments in the middle Okinawa Trough since 30 ka: Constraints from rare earth element compositions[J]. Marine Geology, 2010, 275(1/4): 212−220. [50] Kubota Y, Kimoto K, Tada R, et al. Variations of East Asian summer monsoon since the last deglaciation based on Mg/Ca and oxygen isotope of planktic foraminifera in the northern East China Sea[J]. Paleoceanography, 2010, 25(4): PA4205. [51] Mathur R, Titley S, Barra F, et al. Exploration potential of Cu isotope fractionation in porphyry copper deposits[J]. Journal of Geochemical Exploration, 2009, 102(1): 1−6. doi: 10.1016/j.gexplo.2008.09.004 [52] Santschi P, Höhener P, Benoit G, et al. Chemical processes at the sediment-water interface[J]. Marine Chemistry, 1990, 30: 269−315. doi: 10.1016/0304-4203(90)90076-O [53] Bentahila Y, Othman D B, Luck J M. Strontium, lead and zinc isotopes in marine cores as tracers of sedimentary provenance: A case study around Taiwan orogen[J]. Chemical Geology, 2008, 248(1/2): 62−82. [54] John S G. The marine biogeochemistry of zinc isotopes[D]. Cambridge: Massachusetts Institute of Technology, 2007. -





 下载:
下载: