Characteristics of carbonate system in the Hangzhou Bay: Under the regulation of air-sea exchange and respiration
-
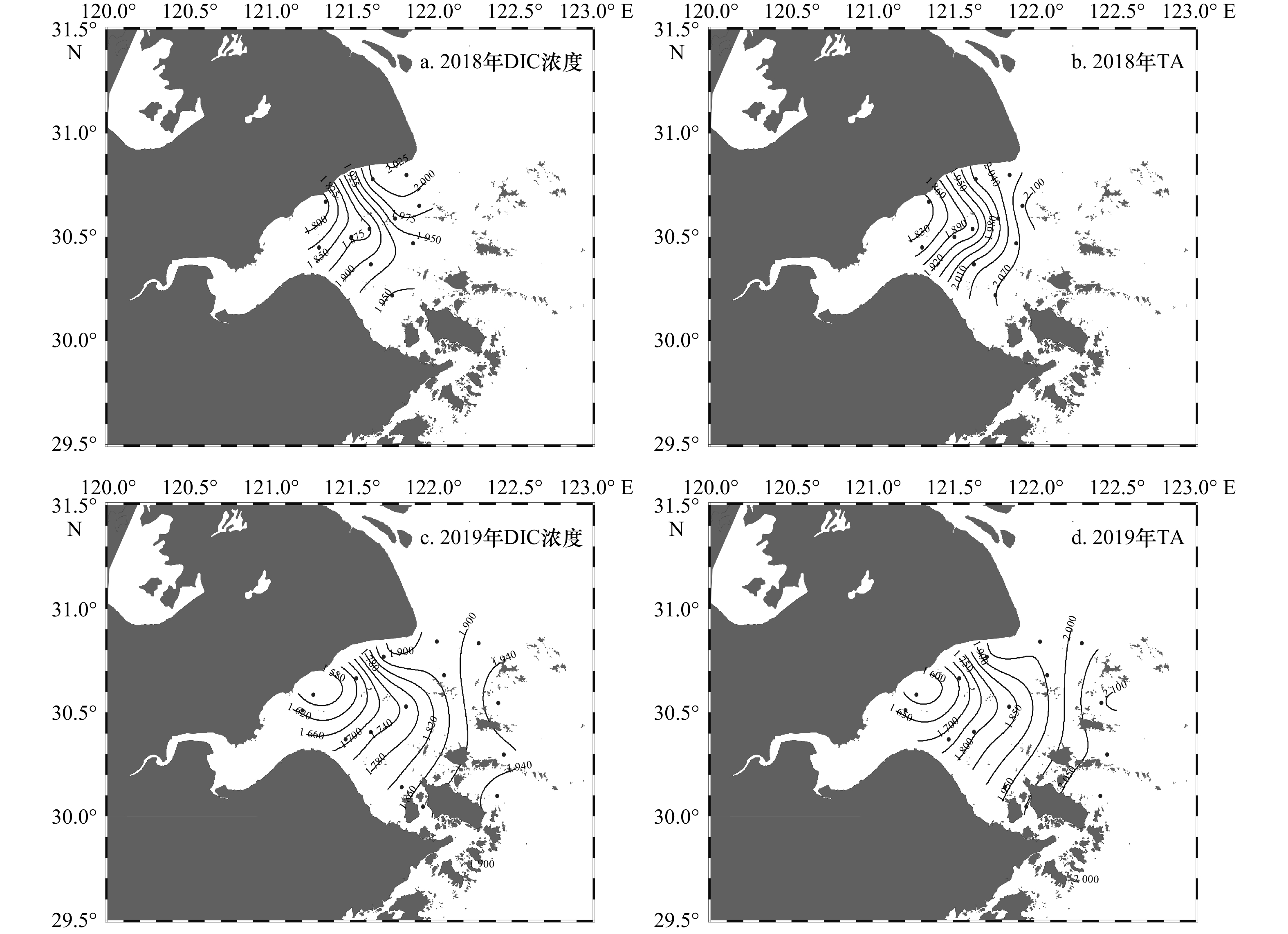
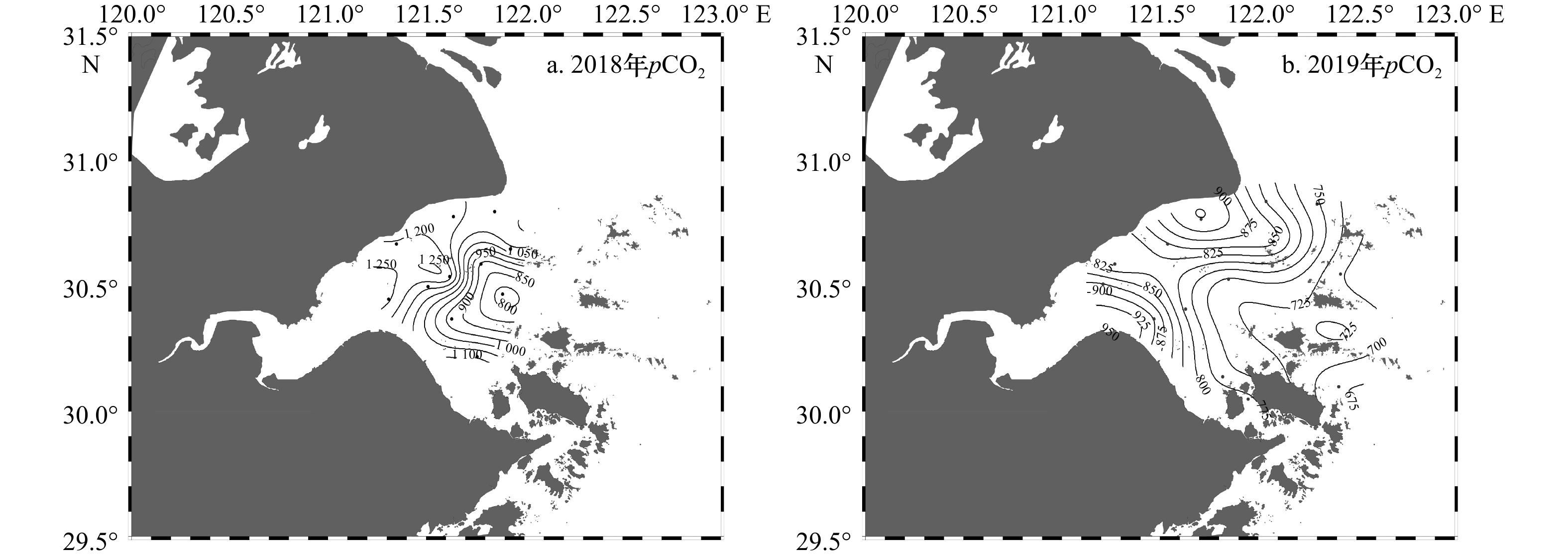
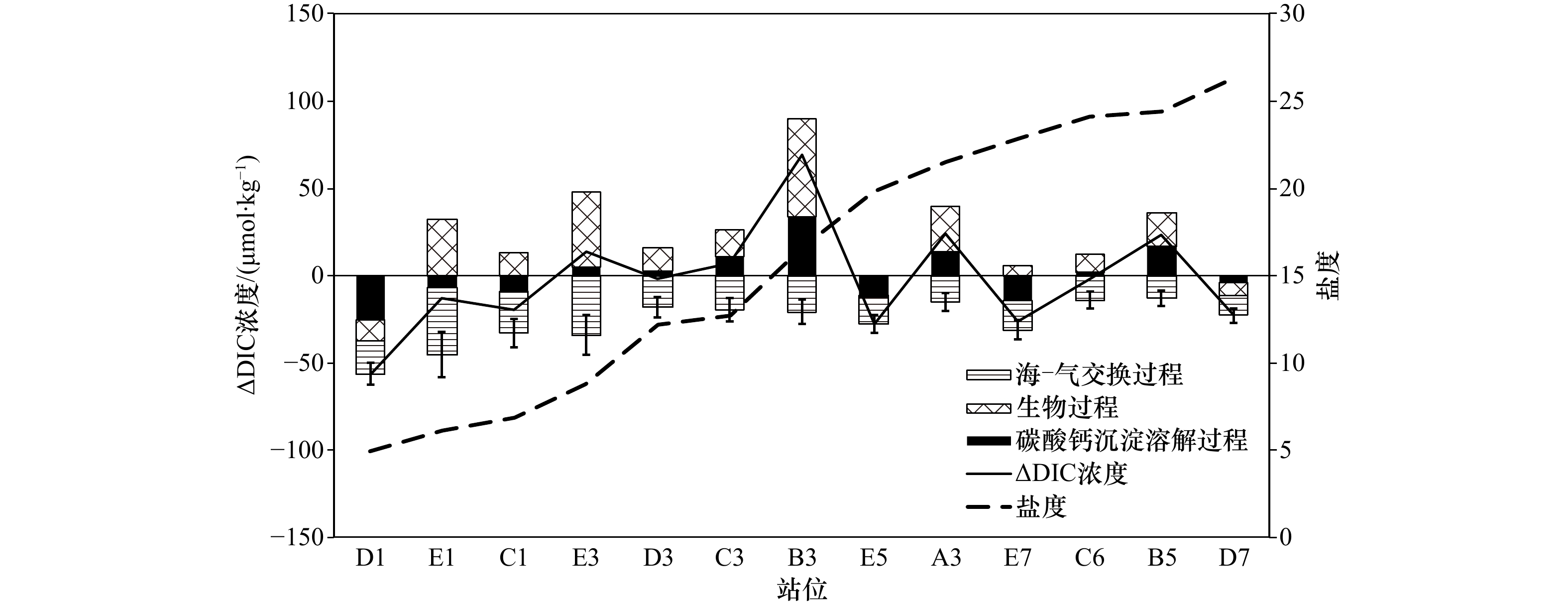
摘要: 杭州湾作为典型的高浑浊度海湾,对其水体碳酸盐体系分布特征的研究相对较少。本文基于两个夏季航次(2018年和2019年)获取的调查资料,阐述夏季杭州湾水体中碳酸盐体系参数的空间分布特征,并进一步分析影响溶解无机碳偏离保守混合作用的主要过程及相对贡献。数据结果表明,杭州湾内表层溶解无机碳浓度与总碱度的变化范围分别为1 553~1 964 μmol/kg和1 577~2 101 μmol/kg,略低于长江口(1 407~2 110 μmol/kg和1 752~2 274 μmol/kg),溶解无机碳浓度和总碱度的空间分布受控于淡水与外海水混合的影响,在潮汐作用下,总体呈现出湾内低,向湾外逐渐升高的趋势。影响表层溶解无机碳非保守混合分布的主要过程中,海−气交换降低溶解无机碳浓度,呼吸作用增加溶解无机碳浓度,两个过程对溶解无机碳浓度变化量的贡献分别为(−42.3±11.7)%与(34.2±14.3)%,净效应呈现为相对平衡的状态。通过计算获得表层海水pCO2的平均值为799 μatm (675~932 μatm),海湾总体表现为大气CO2的源。此外,湾内海水碳酸盐缓冲因子的范围为12.8~23.8,对CO2的缓冲能力弱于邻近东海海水(缓冲因子平均值约为11.9),指示其与外部水体的交换可能会降低附近海域的酸化缓冲能力。相对其他河口/海湾而言,杭州湾内高浊度与强潮汐的特点使其湾内水体的碳酸盐体系分布特征具有区域特殊性。Abstract: As a typical high-turbidity bay, the carbonate systems in the Hangzhou Bay are not well documented. In this paper, the spatial distributions of inorganic carbonate paramenters in the Hangzhou Bay were analyzed based on data collected from two summer surveys in 2018 and 2019. The results showed that dissolved inorganic carbon (DIC) concentration and total alkalinity (TA) in surface layer of the Hangzhou Bay ranged from 1 553 μmol/kg to 1 964 μmol/kg and from 1 577 μmol/kg to 2 101 μmol/kg, respectively, which were lower than that of the Changjiang River Estuary (1 407−2 110 μmol/kg and 1 752−2 274 μmol/kg). The spatial distributions of DIC concentration and TA were controlled by the mixing of fresh water and offshore sea water. They were affected by strong tide, which gradually increased DIC concentration from inner bay to outlet of the bay. Air-sea carbon exchange and biological respiration led to decrease and increase of DIC concentration, with the contributions of (−42.3±11.7)% and (34.2±14.3)%, respectively. Such two compensate processes resulted in a net balanced state. The average surface pCO2 in the Hangzhou Bay was 799 μatm (675−932 μatm), indicating that bay waters were source of atmospheric CO2. The revelle factor in the Hangzhou Bay varied from 12.8 to 23.8, suggesting a weaker CO2 buffering capacity than the adjacent East China Sea (the mean value was 11.9). Compared with other estuaries/gulfs, the characteristics of high turbidity and strong tides in the Hangzhou Bay made the spatial distributions of the carbonate system in the bay water had regional specificity.
-
Key words:
- carbonate systems /
- dissolved inorganic carbon (DIC) /
- distribution /
- Hangzhou Bay
-
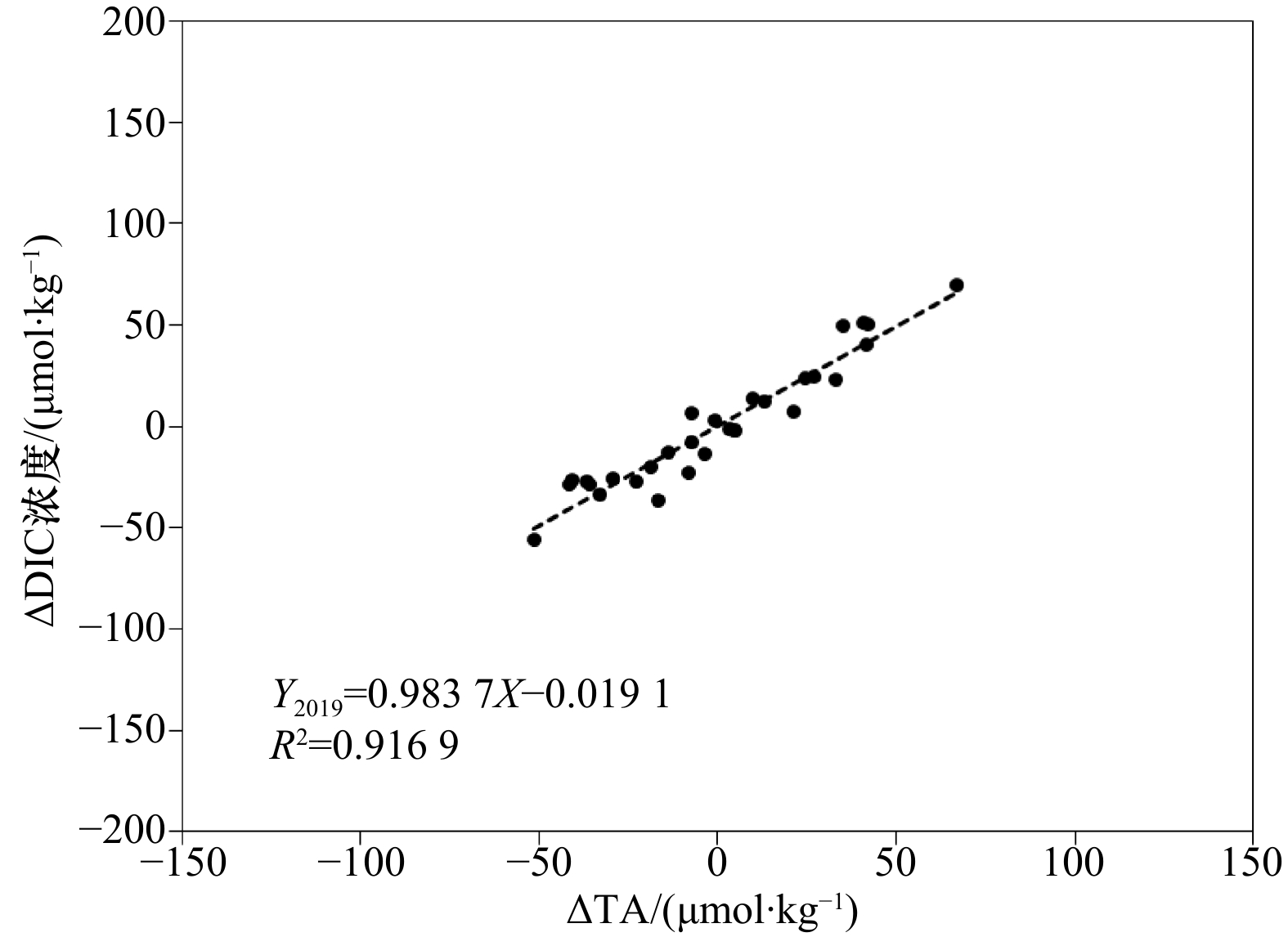
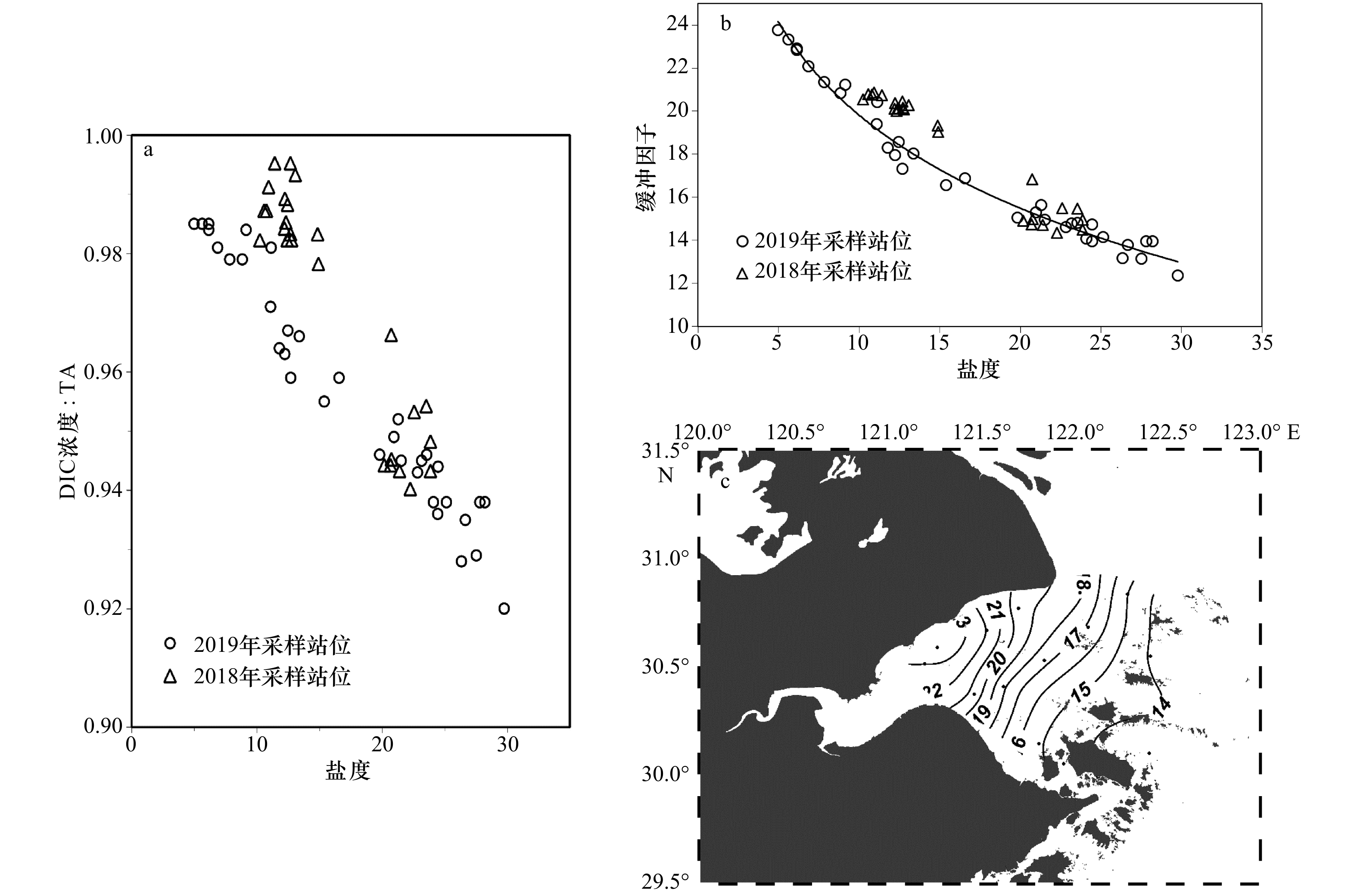
图 5 TA、DIC浓度与盐度之间的相互关系
虚线与直线分别表示2018年、2019年的趋势线;红圈内展示的是北部沿岸高值站位
Fig. 5 The correlation among TA, DIC concentration and salinity
The dotted line and the straight line represent the trend lines of 2018 and 2019, respectively; the high-value stations along the northern coast are shown in the red circle
表 1 航次信息与水文参数
Tab. 1 Cruise information and hydrological parameters
航次名称 采样时间(阳历) 温度/℃ 盐度 深度/m 潮汐 2018年航次 2018年8月12日、2018年8月15日 29.87±0.52 16.02±4.89 8.2±2.1 大潮 2019年航次 2019年8月25−29日 28.45±1.58 17.34±7.85 15.7±12.5 小潮 注:温度、盐度和深度数据为各个航次调查站位的平均值±相对偏差,潮汐以农历推算。 表 2 两个航次表层DIC浓度、TA、pCO2的变化范围
Tab. 2 The variation range of DIC concentration, TA and pCO2 in surface layer of two field cruises
航次年月 DIC浓度/(μmol·kg−1) TA/(μmol·kg−1) pCO2/μatm 2018年8月 1 789~2 015 (1 921) 1 822~2 100 (1 982) 778~1 271 (1 085) 2019年8月 1 553~1 964 (1 805) 1 577~2 101 (1 886) 675~932 (799) 注:DIC浓度、TA为实测值,pCO2为计算值,括号内为平均值。 表 3 7个北半球中高纬度河口/海湾碳酸盐体系的对比
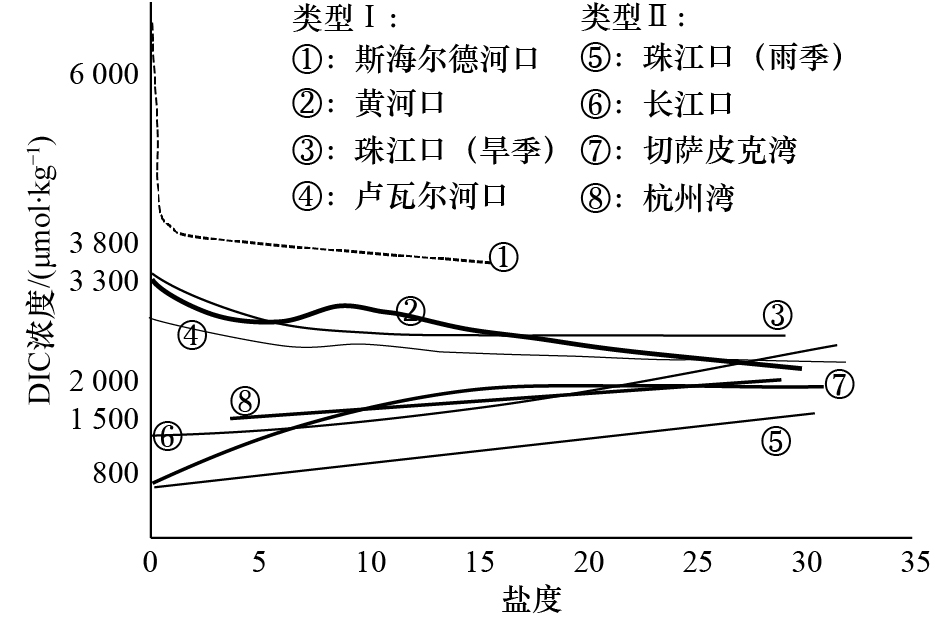
Tab. 3 Comparison of carbonate systems in seven different estuaries and gulfs in the North Hemisphere
河口/海湾 DIC浓度 pCO2/μatm 主要影响因素 数据来源 斯海尔德河口 3 300~7 100 μmol/L 2 200~15 500 呼吸作用 文献[9] 卢瓦尔河口 2 200~2 700 μmol/kg 700~2 900 呼吸作用/
碳酸盐溶解文献[12] 黄河口 2 155~2 927 μmol/L 400~750 初级生产/
碳酸盐析出文献[11] 长江口 1 407~2 110 μmol/kg 177~1 036 初级生产 文献[25, 28] 珠江口 798~1 572 μmol/kg(雨季);
2 744~3 329 μmol/kg(旱季)− 碳酸盐浓度 文献[55] 切萨皮克湾 800~1 900 μmol/kg − 初级生产/呼吸作用 文献[13] 杭州湾 1 553~1 964 μmol/kg 675~932 呼吸作用/海−气交换 本文 注:−代表对应时期数据不可用。 -
[1] Cai Weijun. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration?[J]. Annual Review of Marine Science, 2011, 3: 123−145. doi: 10.1146/annurev-marine-120709-142723 [2] Chen C T A, Huang T H, Chen Y C, et al. Air–sea exchanges of CO2 in the world’s coastal seas[J]. Biogeosciences, 2013, 10(10): 6509−6544. doi: 10.5194/bg-10-6509-2013 [3] Dai Minhan, Cao Zhimian, Guo Xianghui, et al. Why are some marginal seas sources of atmospheric CO2?[J]. Geophysical Research Letters, 2013, 40(10): 2154−2158. doi: 10.1002/grl.50390 [4] Borges A V, Delille B, Frankignoulle M. Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystems counts[J]. Geophysical Research Letters, 2005, 32(14): L14601. [5] Cai Weijun, Dai Minhan, Wang Yongchen. Air-sea exchange of carbon dioxide in ocean margins: a province-based synthesis[J]. Geophysical Research Letters, 2006, 33(12): L12603. doi: 10.1029/2006GL026219 [6] Cai Weijun. Riverine inorganic carbon flux and rate of biological uptake in the Mississippi River plume[J]. Geophysical Research Letters, 2003, 30(2): 1032. [7] Dürr H H, Laruelle G G, Van Kempen C M, et al. Worldwide typology of nearshore coastal systems: defining the estuarine filter of river inputs to the oceans[J]. Estuaries and Coasts, 2011, 34(3): 441−458. doi: 10.1007/s12237-011-9381-y [8] Guo Xianghui, Cai Weijun, Huang W J, et al. Carbon dynamics and community production in the Mississippi River plume[J]. Limnology and Oceanography, 2012, 57(1): 1−17. doi: 10.4319/lo.2012.57.1.0001 [9] Hellings L, Dehairs F, Van Damme S, et al. Dissolved inorganic carbon in a highly polluted estuary (the Scheldt)[J]. Limnology and Oceanography, 2001, 46(6): 1406−1414. doi: 10.4319/lo.2001.46.6.1406 [10] Cai Weijun, Guo Xianghui, Chen C T A, et al. A comparative overview of weathering intensity and $ {\rm{HCO}}_3^- $ flux in the world’s major rivers with emphasis on the Changjiang, Huanghe, Zhujiang (Pearl) and Mississippi Rivers[J]. Continental Shelf Research, 2008, 28(12): 1538−1549. doi: 10.1016/j.csr.2007.10.014[11] Liu Zhiyuan, Zhang Longjun, Cai Weijun, et al. Removal of dissolved inorganic carbon in the Yellow River Estuary[J]. Limnology and Oceanography, 2014, 59(2): 413−426. doi: 10.4319/lo.2014.59.2.0413 [12] Abril G, Etcheber H, Delille B, et al. Carbonate dissolution in the turbid and eutrophic Loire estuary[J]. Marine Ecology Progress Series, 2003, 259: 129−138. doi: 10.3354/meps259129 [13] Su Jianzhong, Cai Weijun, Brodeur J, et al. Chesapeake Bay acidification buffered by spatially decoupled carbonate mineral cycling[J]. Nature Geoscience, 2020, 13(6): 441−447. doi: 10.1038/s41561-020-0584-3 [14] Millero F J, Hiscock W T, Huang F, et al. Seasonal variation of the carbonate system in Florida Bay[J]. Bulletin of Marine Science, 2001, 68(1): 101−123. [15] Wang H, Xue Hongchao. China-U. S. Joint Muddy Coast Research, Part 1, a review of hydrological and sedimentary processes in Hangzhou Bay, China[R]. Gainesville: Coastal Engineering Research Center, 1990. [16] Chen Shenliang, Zhang Guoan, Yang Shilun, et al. Temporal variations of fine suspended sediment concentration in the Changjiang River estuary and adjacent coastal waters, China[J]. Journal of Hydrology, 2006, 331(1/2): 137−145. [17] Xie Dongfeng, Wang Zhengbing, Gao Shu, et al. Modeling the tidal channel morphodynamics in a macro-tidal embayment, Hangzhou Bay, China[J]. Continental Shelf Research, 2009, 29(15): 1757−1767. doi: 10.1016/j.csr.2009.03.009 [18] 高生泉, 陈建芳, 金海燕, 等. 杭州湾及邻近水域营养盐的时空分布与富营养化特征[J]. 海洋学研究, 2011, 29(3): 36−47. doi: 10.3969/j.issn.1001-909X.2011.03.005Gao Shengquan, Chen Jianfang, Jin Haiyan, et al. Characteristics of nutrients and eutrophication in the Hangzhou Bay and its adjacent waters[J]. Journal of Marine Sciences, 2011, 29(3): 36−47. doi: 10.3969/j.issn.1001-909X.2011.03.005 [19] Zhou Weihua, Yin Kedong, Long Aimin, et al. Spatial-temporal variability of total and size-fractionated phytoplankton biomass in the Yangtze River Estuary and adjacent East China Sea coastal waters, China[J]. Aquatic Ecosystem Health & Management, 2012, 15(2): 200−209. [20] Wu Bin, Jin Haiyan, Gao Shengquan, et al. Nutrient budgets and recent decadal variations in a highly eutrophic estuary: Hangzhou Bay, China[J]. Journal of Coastal Research, 2020, 36(1): 63−71. [21] Zhai Weidong, Dai Minhan, Guo Xianghui. Carbonate system and CO2 degassing fluxes in the inner estuary of Changjiang (Yangtze) River, China[J]. Marine Chemistry, 2007, 107(3): 342−356. doi: 10.1016/j.marchem.2007.02.011 [22] Chen C T A, Zhai Weidong, Dai Minhan. Riverine input and air-sea CO2 exchanges near the Changjiang (Yangtze River) Estuary: status quo and implication on possible future changes in metabolic status[J]. Continental Shelf Research, 2008, 28(12): 1476−1482. doi: 10.1016/j.csr.2007.10.013 [23] Chou Wenchen, Gong G C, Sheu D D, et al. Surface distributions of carbon chemistry parameters in the East China Sea in summer 2007[J]. Journal of Geophysical Research: Oceans, 2009, 114(C7): C07026. [24] Li Xuegang, Song Jinming, Yuan Huamao, et al. CO2 flux and seasonal variability in the turbidity maximum zone and surrounding area in the Changjiang River estuary[J]. Chinese Journal of Oceanology and Limnology, 2015, 33(1): 222−232. doi: 10.1007/s00343-014-3282-4 [25] Xiong Tianqi, Liu Pengfei, Zhai Weidong, et al. Export flux, biogeochemical effects, and the fate of a terrestrial carbonate system: from Changjiang (Yangtze River) estuary to the East China Sea[J]. Earth and Space Science, 2019, 6(11): 2115−2141. doi: 10.1029/2019EA000679 [26] Gao Xuelu, Song Jinming, Li Xuegang, et al. pCO2 and carbon fluxes across sea-air interface in the Changjiang Estuary and Hangzhou Bay[J]. Chinese Journal of Oceanology and Limnology, 2008, 26(3): 289−295. doi: 10.1007/s00343-008-0289-8 [27] Zhai Weidong, Dai Minhan. On the seasonal variation of air-sea CO2 fluxes in the outer Changjiang (Yangtze River) Estuary, East China Sea[J]. Marine Chemistry, 2009, 117(1/4): 2−10. [28] Yu Peisong, Zhang Haisheng, Zheng Minhui, et al. The partial pressure of carbon dioxide and air-sea fluxes in the Changjiang River Estuary and adjacent Hangzhou Bay[J]. Acta Oceanologica Sinica, 2013, 32(6): 13−17. doi: 10.1007/s13131-013-0320-6 [29] Guo Xianghui, Zhai Weidong, Dai Minhan, et al. Air-sea CO2 fluxes in the East China Sea based on multiple-year underway observations[J]. Biogeosciences, 2015, 12(18): 5495−5514. doi: 10.5194/bg-12-5495-2015 [30] Liu Qian, Dong Xu, Chen Jinshun, et al. Diurnal to interannual variability of sea surface pCO2 and its controls in a turbid tidal-driven nearshore system in the vicinity of the East China Sea based on buoy observations[J]. Marine Chemistry, 2019, 216: 103690. doi: 10.1016/j.marchem.2019.103690 [31] 李福荣, 陈国华, 陈景. 杭州湾海水碱度研究[J]. 青岛海洋大学学报, 1999(S1): 55−62.Li Furong, Chen Guohua, Chen Jing. The alkalinity of seawater in Hangzhou Bay[J]. Journal of Ocean University of Qingdao, 1999(S1): 55−62. [32] 高学鲁, 宋金明, 李学刚, 等. 长江口及杭州湾邻近海域夏季表层海水中的溶解无机碳[J]. 海洋科学, 2008, 32(4): 61−67.Gao Xuelu, Song Jinming, Li Xuegang, et al. Dissolved inorganic carbon in surface waters around the Changjiang Estuary and Hangzhou Bay in summer[J]. Marine Sciences, 2008, 32(4): 61−67. [33] Dickson A G, Sabine C L, Christian J R. Guide to best practices for ocean CO2 measurements[R]. Sidney: North Pacific Marine Science Organization, 2007: 1−191. [34] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. GB/T 12763.4−2007, 《海洋调查规范》第4部分: 海水化学要素调查[S]. 北京: 中国标准出版社, 2008.General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People’s Republic of China. GB/T 12763.4−2007, Specifications for oceanographic survey—Part 4: survey of chemical parameters in sea water[S]. Beijing: Standards Press of China, 2008. [35] Cai Weijun, Hu Xinping, Huang W J, et al. Acidification of subsurface coastal waters enhanced by eutrophication[J]. Nature Geoscience, 2011, 4(11): 766−770. doi: 10.1038/ngeo1297 [36] Millero F J, Graham T B, Huang Fen, et al. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature[J]. Marine Chemistry, 2006, 100(1/2): 80−94. [37] Dickson A G. Standard potential of the reaction: AgCl(s)+12H2(g)=Ag(s)+HCl(aq), and the standard acidity constant of the ion ${\rm{HSO}}_4^- $ in synthetic sea water from 273.15 to 318.15 K[J]. The Journal of Chemical Thermodynamics, 1990, 22(2): 113−127. doi: 10.1016/0021-9614(90)90074-Z[38] Benson B B, Krause Jr D. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere[J]. Limnology and Oceanography, 1984, 29(3): 620−632. doi: 10.4319/lo.1984.29.3.0620 [39] Wanninkhof R. Relationship between wind speed and gas exchange over the ocean revisited[J]. Limnology and Oceanography: Methods, 2014, 12(6): 351−362. doi: 10.4319/lom.2014.12.351 [40] Wu Hui, Zhu Jianrong, Shen Jian, et al. Tidal modulation on the Changjiang River plume in summer[J]. Journal of Geophysical Research: Oceans, 2011, 116(C8): C08017. [41] 倪勇强, 耿兆铨, 朱军政. 杭州湾水动力特性研讨[J]. 水动力学研究与进展, 2003, 18(4): 439−445.Ni Yongqiang, Geng Zhaoquan, Zhu Junzheng. Study on characteristic of hydrodynamics in Hangzhou Bay[J]. Journal of Hydrodynamics, 2003, 18(4): 439−445. [42] Cai Weijun, Dai Minhan, Wang Yongchen, et al. The biogeochemistry of inorganic carbon and nutrients in the Pearl River estuary and the adjacent Northern South China Sea[J]. Continental Shelf Research, 2004, 24(12): 1301−1319. doi: 10.1016/j.csr.2004.04.005 [43] Ortega T, Ponce R, Forja J, et al. Benthic fluxes of dissolved inorganic carbon in the Tinto—Odiel system (SW of Spain)[J]. Continental Shelf Research, 2008, 28(3): 458−469. doi: 10.1016/j.csr.2007.10.004 [44] Redfield A C, Ketchum B H, Richards F A. The influence of organisms on the composition of sea-water[M]//Hill M N. The Composition of Seawater: Comparative and Descriptive Oceanography. New York: Interscience Publishers, 1963, 2: 26−77. [45] Frankignoulle M, Abril G, Borges A, et al. Carbon dioxide emission from European estuaries[J]. Science, 1998, 282(5388): 434−436. doi: 10.1126/science.282.5388.434 [46] Abril G, Etcheber H, Borges A V, et al. Excess atmospheric carbon dioxide transported by rivers into the Scheldt Estuary[J]. Comptes Rendus de l’Académie des Sciences-Series IIA-Earth and Planetary Science, 2000, 330(11): 761−768. [47] 吴婷. 杭州湾水龄和滞留时间特性研究[D]. 杭州: 浙江大学, 2016.Wu Ting. Characteristics of water age and residence time in Hangzhou Bay[D]. Hangzhou: Zhejiang University, 2016. [48] Middelburg J, Soetaert K, Hagens M. Ocean alkalinity, buffering and biogeochemical processes[J]. Reviews of Geophysics, 2019, 18: 41−46. [49] Xue Liang, Cai Weijun. Total alkalinity minus dissolved inorganic carbon as a proxy for deciphering ocean acidification mechanisms[J]. Marine Chemistry, 2020, 222: 103791. doi: 10.1016/j.marchem.2020.103791 [50] Broecker W S, Takahashi T, Simpson H J, et al. Fate of fossil fuel carbon dioxide and the global carbon budget[J]. Science, 1979, 206(4417): 409−418. doi: 10.1126/science.206.4417.409 [51] Egleston E S, Sabine C L, Morel F M M. Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity[J]. Global Biogeochemical Cycles, 2010, 24(1): GB1002. [52] Zhai Weidong, Chen Jianfang, Jin Haiyan, et al. Spring carbonate chemistry dynamics of surface waters in the northern East China Sea: water mixing, biological uptake of CO2, and chemical buffering capacity[J]. Journal of Geophysical Research Oceans, 2014, 119(9): 5638−5653. doi: 10.1002/2014JC009856 [53] Xiong Tianqi, Wei Qinsheng, Zhai Weidong, et al. Comparing subsurface seasonal deoxygenation and acidification in the Yellow Sea and Northern East China Sea along the North-to-South latitude gradient[J]. Frontiers in Marine Science, 2020, 7: 686. doi: 10.3389/fmars.2020.00686 [54] 刘新成, 卢永金, 潘丽红, 等. 长江口和杭州湾潮流数值模拟及水体交换的定量研究[J]. 水动力学研究与进展, 2006, 21(2): 171−180.Liu Xincheng, Lu Yongjin, Pan Lihong, et al. Tidal current numerical simulating and water exchange research in Yangtze Estuary and Hangzhou Bay[J]. Journal of Hydrodynamics, 2006, 21(2): 171−180. [55] Guo Xianghui, Cai Weijun, Zhai Weidong, et al. Seasonal variations in the inorganic carbon system in the Pearl River (Zhujiang) estuary[J]. Continental Shelf Research, 2008, 28(12): 1424−1434. doi: 10.1016/j.csr.2007.07.011 -





 下载:
下载: