Effects of low temperature stress on the expression of genes related to lipid metabolism of juvenile cobia, Rachycentron canadum
-
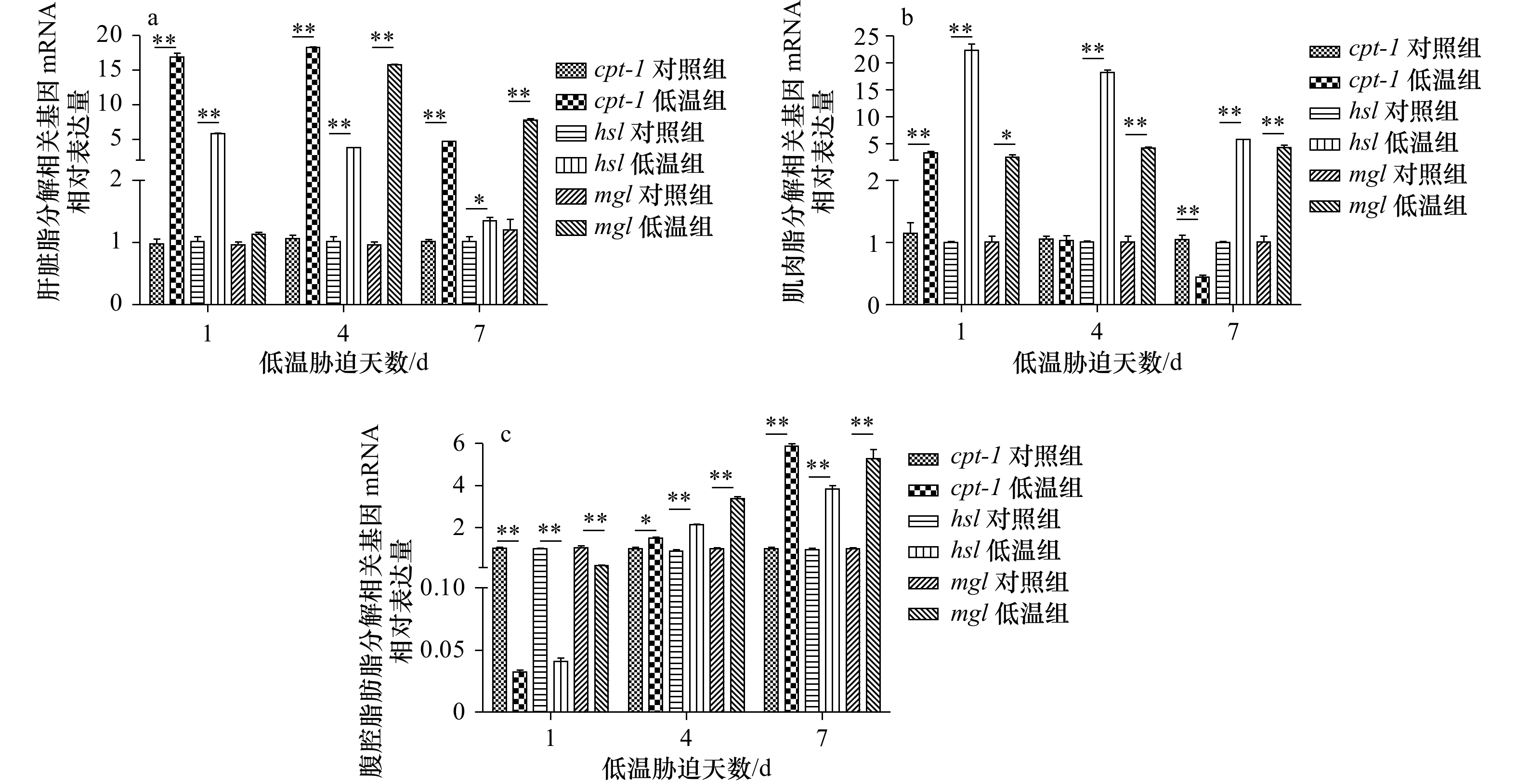
摘要: 将两组军曹鱼(Rachycentron canadum)幼鱼分别在常温(30.5±1.0)℃和低温(20.0±0.5)℃环境下饲养7 d,并于第1天、第4天、第7天3个时间点采集肝脏、肌肉和腹腔脂肪,利用实时荧光定量PCR(qRT-PCR)技术分析5个脂代谢相关基因的表达变化情况,以探究低温胁迫对军曹鱼脂质合成与分解代谢的影响。结果显示,第1天时,肝脏的肉碱脂酰基转移酶-1基因(cpt-1)、脂肪激素敏感脂肪酶基因(hsl)以及肌肉的cpt-1、hsl、单酰基甘油酯酶基因(mgl)等显著上调(p<0.05),肝脏、肌肉的乙酰辅酶A羧化酶基因(acc)和脂肪酸合成酶基因(fas)以及腹腔脂肪(IPF)5个脂代谢相关基因表达均显著下调(p<0.05);第4天时,肝脏的cpt-1、hsl、mgl和肌肉的hsl、mgl、acc、fas以及IPF的cpt-1、hsl、mgl、acc等表达上调(p<0.05),肝脏的acc、fas表达
显著下调(p<0.05);第7天时,肝脏和IPF的cpt-1、hsl、mgl、acc和肌肉的hsl、mgl、acc等表达上调(p<0.05),肌肉cpt-1 和肝脏fas表达显著下调(p<0.05)。结果表明,军曹鱼幼鱼在低温胁迫前期通过抑制脂合成代谢,促进肝脏和肌肉中的脂质水解,通过抑制腹腔脂肪的脂质分解来响应低温胁迫;在低温胁迫后期,军曹鱼幼鱼脂合成和分解代谢均显著提高,且利用脂肪酸提供能量的主要组织由前期的肝脏和肌肉转变为肝脏和腹腔脂肪。 Abstract: In order to explore the effects of low temperature stress on the expression of genes related to lipid synthesis and catabolism in cobia (Rachycentron canadum), the experiment set up a normal temperature group (30.5±1.0)°C and a low temperature group (20.0±0.5)°C, and used real-time fluorescent quantitative PCR (qRT-PCR) to analyze the expression levels of 5 target genes in liver, muscle and intraperitoneal fat (IPF). The results showed that at 1 d, the expression ofcarnitine palmitoyl transferase-1 and hormone-sensitive lipase genes of liver, carnitine palmitoyl transferase-1, hormone-sensitive lipase and monoacylglycerol lipase genes of muscle were up-regulated (p<0.05), acetyl-CoA carboxylase and fatty acid synthase genes of liver, muscle and 5 lipid metabolism related genes of IPF were significantly down-regulated (p<0.05); at 4 d, the expression of carnitine palmitoyl transferase-1, hormone-sensitive lipase and monoacylglycerol lipase genes of liver, and hormone-sensitive lipase, monoacylglycerol lipase, acetyl-CoA carboxylase, fatty acid synthase genes of muscle and carnitine palmitoyl transferase-1, hormone-sensitive lipase, monoacylglycerol lipase, acetyl-CoA carboxylase genes of IPF were up-regulated (p<0.05), acetyl-CoA carboxylase and fatty acid synthase gene of liver were down-regulated (p<0.05); at 7 d, the expressions of carnitine palmitoyl transferase-1, hormone-sensitive lipase, monoacylglycerol lipase, acetyl-CoA carboxylase genes of liver and IPF, and hormone-sensitive lipase, monoacylglycerol lipase, acetyl-CoA carboxylase genes of muscle were up-regulated (p<0.05), carnitine palmitoyl transferase-1 gene of muscle and fatty acid synthase genes of liver were down-regulated (p<0.05). The results showed that cobia responded to low temperature stress by inhibiting lipid synthesis and metabolism, promoting lipid hydrolysis in the liver and muscle, and inhibiting the lipid hydrolysis of IPF in the early stage of low temperature stress; in the late period of low temperature stress, cobia lipid synthesis and catabolism were significantly increased, and the main tissue that used fatty acids to provide energy was transformed from the liver and muscle to liver and IPF. -
Key words:
- low temperature stress /
- lipid metabolism /
- gene expression /
- Rachycentron canadum /
- real-time qRT-PCR
-
图 1 低温胁迫下军曹鱼幼鱼3个脂分解相关基因表达量
*表示低温组与同期对照组之间差异显著(p<0.05);**表示差异极显著(p<0.01)
Fig. 1 Expression of three lipolysis-related genes in juvenile cobia under low temperature stress
* indicates a significant difference (p<0.05); ** indicates extremely significant difference (p<0.01) compared with the control group in the same period
图 2 低温胁迫下军曹鱼幼鱼3个脂合成相关基因表达量
*表示低温组与同期对照组之间差异显著(p<0.05);**表示差异极显著(p<0.01)
Fig. 2 Expression of three lipid synthesis-related genes in juvenile cobia under low temperature stress
* indicates a significant difference (p<0.05) ; ** indicates extremely significant difference (p<0.01) compared with the control group in the same period
表 1 本实验引物序列
Tab. 1 The primers used in the experiment
引物名称 序列(5′-3′) 用途 acc-F TCGCCAGTCTCCCAACTCCTAT acc荧光定量引物 acc-R ACCTGTCCACCTCCTCCTTCAT acc荧光定量引物 fas-F AGCATCCTGTATCGCCCGTTTGA fas荧光定量引物 fas-R GTCGGTCCTGTGGGTCTCCTTGT fas荧光定量引物 hsl-F AGCAGTCTGGTTTGGGTTTGGC hsl荧光定量引物 hsl-R AGGTTCTGGGTAATGCGTTCA hsl荧光定量引物 cpt-1-F TACCGCTTGGCTATGACTGGAC cpt-1荧光定量引物 cpt-1-R TTGCTGGAGATGTGGAAGTTGATG cpt-1荧光定量引物 mgl-F CACTGCGACCTTTGACCTCTTTG mgl荧光定量引物 mgl-R AACCATCCTTCTGGGCGTAATC mgl荧光定量引物 β-actin-F AGGGAAATTGTGCGTGAC 内参基因荧光定量引物 β-actin-R AGGCAGCTCGTAGCTCTT 内参基因荧光定量引物 注:acc为乙酰辅酶A羧化酶基因;fas为脂肪酸合成酶基因;hsl为脂肪激素敏感脂肪酶基因;cpt-1为肉碱脂酰基转移酶-1基因;mgl为单酰基甘油酯酶基因; β-actin为β-肌动蛋白基因。 -
[1] 管敏, 张厚本, 王龙, 等. 急性低温胁迫对史氏鲟幼鱼抗氧化和免疫指标的影响[J]. 淡水渔业, 2018, 48(6): 17−22. doi: 10.3969/j.issn.1000-6907.2018.06.003Guan Min, Zhang Houben, Wang Long, et al. The effects of acute low temperature stress on antioxidative and immune indices of juvenile Amur sturgeon (Acipenser schrenckii)[J]. Freshwater Fisheries, 2018, 48(6): 17−22. doi: 10.3969/j.issn.1000-6907.2018.06.003 [2] 冉皓宇, 陈良标. 低温驯化对斑马鱼胚胎发育和mtDNA拷贝数的影响[J]. 生物学杂志, 2019, 36(5): 16−20. doi: 10.3969/j.issn.2095-1736.2019.05.016Ran Haoyu, Chen Liangbiao. Effects of cold acclimation on the embryogenesis and mtDNA copy number in zebrafish embryos[J]. Journal of Biology, 2019, 36(5): 16−20. doi: 10.3969/j.issn.2095-1736.2019.05.016 [3] 张宇航, 高扬, 李文红, 等. 低温停食和复温后投喂频率对奥尼罗非鱼幼鱼生长的影响[J]. 西南农业学报, 2020, 33(9): 2125−2131.Zhang Yuhang, Gao Yang, Li Wenhong, et al. Effects of feeding frequency after food deprivation with low temperature and rewarming on growth of hybrid tilapia juvenile (Oreochromis niloticus×O.aureus)[J]. Southwest China Journal of Agricultural Sciences, 2020, 33(9): 2125−2131. [4] Ibarz A, Beltrán M, Fernández-Borràs J, et al. Alterations in lipid metabolism and use of energy depots of gilthead sea bream (Sparus aurata) at low temperatures[J]. Aquaculture, 2007, 262(2/4): 470−480. [5] He J, Qiang J, Yang H, et al. Changes in the fatty acid composition and regulation of antioxidant enzymes and physiology of juvenile genetically improved farmed tilapia Oreochromis niloticus (L.), subjected to short-term low temperature stress[J]. Journal of Thermal Biology, 2015, 53: 90−97. doi: 10.1016/j.jtherbio.2015.08.010 [6] Sun Junlong, Zhao Liulan, Cui Can, et al. Influence of long-term temperature stress on respiration frequency, Na+/K+-ATPase activity, and lipid metabolism in common carp (Cyprinus carpio)[J]. Journal of Thermal Biology, 2019, 83: 165−171. doi: 10.1016/j.jtherbio.2019.05.009 [7] Sun Zhenzhu, Tan Xiaohong, Liu Qingying, et al. Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress[J]. Aquaculture, 2019, 498: 545−555. doi: 10.1016/j.aquaculture.2018.08.051 [8] 方玲玲. 卵形鲳鲹PPARα及CPTⅠ基因的克隆及不同条件对其表达影响的分析[D]. 湛江: 广东海洋大学, 2016.Fang Lingling. Molecular cloning and expression under the different conditions of peroxisome proliferator activated receptors-α and Carnitine PalmitoyltransferaseⅠ in Trachinotus ovatus[D]. Zhanjiang: Guangdong Ocean University, 2016. [9] Mininni A N, Milan M, Ferraresso S, et al. Liver transcriptome analysis in gilthead sea bream upon exposure to low temperature[J]. BMC Genomics, 2014, 15(1): 765. doi: 10.1186/1471-2164-15-765 [10] 萧培珍. 日粮中添加水飞蓟素对草鱼脂质代谢的影响及其机制研究[D]. 杨凌: 西北农林科技大学, 2017.Xiao Peizhen. Effect of dietary silymarin on lipid metabolism of grass carp (Ctenopharygodon idellus)[D]. Yangling: Northwest A&F University, 2017. [11] Lampidonis A D, Rogdakis E, Voutsinas G E, et al. The resurgence of hormone-sensitive lipase (HSL) in mammalian lipolysis[J]. Gene, 2011, 477(1/2): 1−11. [12] Blankman J L, Simon G M, Cravatt B F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol[J]. Chemistry & Biology, 2007, 14(12): 1347−1356. [13] Long J Z, Li Weiwei, Booker L, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects[J]. Nature Chemical Biology, 2009, 5(1): 37−44. doi: 10.1038/nchembio.129 [14] Mannaerts G P, Debeer L J, Thomas J, et al. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats[J]. Journal of Biological Chemistry, 1979, 254(11): 4585−4595. doi: 10.1016/S0021-9258(17)30051-0 [15] 杨健, 陈刚, 黄建盛, 等. 温度和盐度对军曹鱼幼鱼生长与抗氧化酶活性的影响[J]. 广东海洋大学学报, 2007, 27(4): 25−29. doi: 10.3969/j.issn.1673-9159.2007.04.006Yang Jian, Chen Gang, Huang Jiansheng, et al. Effects of temperature and salinity on the growth and activities of antioxidant enzymes of cobia (Rachycentron canadum) juveniles[J]. Journal of Guangdong Ocean University, 2007, 27(4): 25−29. doi: 10.3969/j.issn.1673-9159.2007.04.006 [16] 孙丽华, 陈浩如. 温度和体质量对军曹鱼生长及氮收支的影响[J]. 水产学报, 2013, 37(10): 1527−1534. doi: 10.3724/SP.J.1231.2013.38597Sun Lihua, Chen Haoru. Effects of water temperature and fish size on growth and nitrogen budget of cobia (Rachycentron canadum)[J]. Journal of Fisheries of China, 2013, 37(10): 1527−1534. doi: 10.3724/SP.J.1231.2013.38597 [17] Ackman R G. Fish lipids, Part 1[M]. Farnham, Surrey: Fishing News Books Ltd., 1980: 86−103. [18] Schmittgen T D, Livak K J. Analyzing real-time PCR data by the comparative CT method[J]. Nature Protocols, 2008, 3(6): 1101−1108. doi: 10.1038/nprot.2008.73 [19] Wen Bin, Jin Shirong, Chen Zaizhong, et al. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics[J]. Science of the Total Environment, 2018, 640−641: 1372−1381. doi: 10.1016/j.scitotenv.2018.05.401 [20] Dietrich M A, Hliwa P, Adamek M, et al. Acclimation to cold and warm temperatures is associated with differential expression of male carp blood proteins involved in acute phase and stress responses, and lipid metabolism[J]. Fish and Shellfish Immunology, 2018, 76: 305−315. doi: 10.1016/j.fsi.2018.03.018 [21] Mateus A P, Costa R, Gisbert E, et al. Thermal imprinting modifies bone homeostasis in cold challenged sea bream (Sparus aurata)[J]. Journal of Experimental Biology, 2017, 220: 3442−3454. doi: 10.1242/jeb.156174 [22] Lu Dongliang, Ma Qiang, Wang Jing, et al. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy[J]. The Journal of Physiology, 2019, 597(6): 1585−1603. doi: 10.1113/JP277091 [23] 邓伟. 温度胁迫对多鳞白甲鱼AMPK介导的能量稳态及脂肪酸代谢的影响[D]. 杨凌: 西北农林科技大学, 2019.Deng Wei. Influence of temperature stress on the AMPK-mediated energy homeostasis and fatty acid metabolism in Onychostoma macrolepis[D]. Yangling: Northwest A&F University, 2019. [24] 林超, 柳军, 孙宏斌. 脂质生物合成的转录调控、相关靶标确证及新药发现[J]. 中国科学: 化学, 2015, 45(9): 923−936. doi: 10.1360/N032015-00114Lin Chao, Liu Jun, Sun Hongbin. Transcriptional regulation of lipid biosynthesis, related target validation and drug discovery[J]. Scientia Sinica Chimica, 2015, 45(9): 923−936. doi: 10.1360/N032015-00114 [25] 严媛, 程汉良, 许建和, 等. 草鱼乙酰辅酶A羧化酶β基因全长cDNA分子克隆与表达分析[J]. 动物营养学报, 2018, 30(5): 1827−1836. doi: 10.3969/j.issn.1006-267x.2018.05.025Yan Yuan, Cheng Hanliang, Xu Jianhe, et al. Molecular cloning of acetyl-CoA carboxylase β full-length cDNA from grass carp (Ctenopharyngodon idella) and expression analysis[J]. Chinese Journal of Animal Nutrition, 2018, 30(5): 1827−1836. doi: 10.3969/j.issn.1006-267x.2018.05.025 [26] 杨文平, 王爱民, 於叶兵, 等. 梭鱼脂肪酸合成酶基因部分片段的克隆和表达分析[J]. 江苏农业科学, 2017, 45(23): 49−54.Yang Wenping, Wang Aimin, Yu Yebin, et al. Cloning and expression analysis of partial fragments of fatty acid synthase gene from Liza haematocheila[J]. Jiangsu Agricultural Sciences, 2017, 45(23): 49−54. [27] McGarry J D, Brown H F. The mitochondrial carnitine palmitoyltransferase system—From concept to molecular analysis[J]. European Journal of Biochemistry, 1997, 244(1): 1−14. doi: 10.1111/j.1432-1033.1997.00001.x [28] 李亮, 程彦伟. 乙酰辅酶A羧化酶在治疗肥胖中的潜在作用[J]. 生命的化学, 2007, 27(2): 180−182. doi: 10.3969/j.issn.1000-1336.2007.02.030Li Liang, Cheng Yanwei. The potential effects of Acetyl-CoA carboxylase in curing obesity[J]. Chemistry of Life, 2007, 27(2): 180−182. doi: 10.3969/j.issn.1000-1336.2007.02.030 -




 下载:
下载:

