Transcription level of immune related genes of juvenile cobia (Rachycentron canadum) under hypoxia stress
-
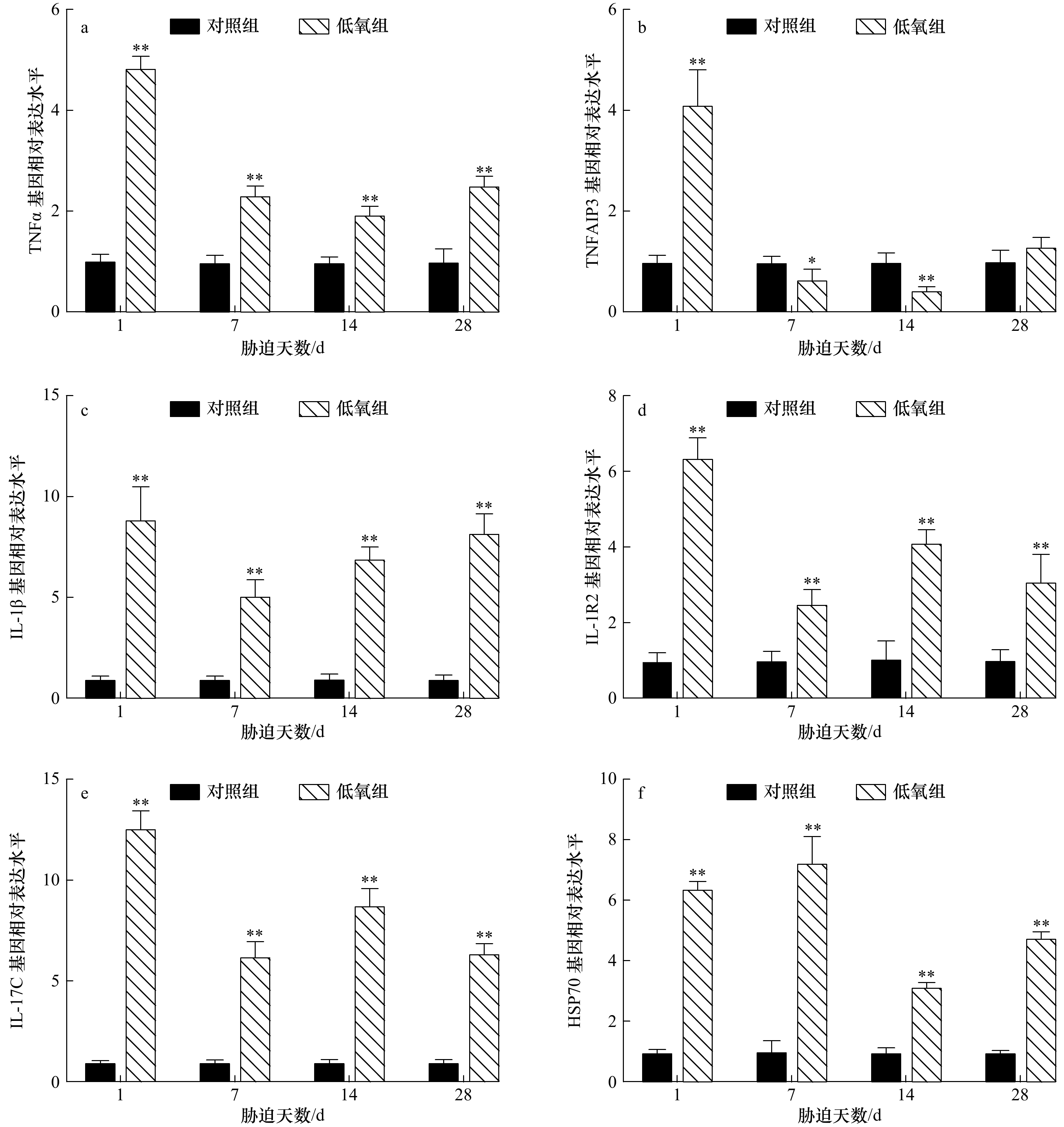
摘要: 为研究低氧胁迫对军曹鱼幼鱼免疫功能的影响,将幼鱼暴露于溶氧浓度为(3.15±0.21) mg/L的水体28 d,测定不同时间点肿瘤坏死因子α (TNFα)、肿瘤坏死因子α诱导蛋白3 (TNFAIP3)、白细胞介素1β (IL-1β)、白细胞介素1受体2 (IL-1R2)、白细胞介素17C (IL-17C)和热休克蛋白70 (HSP70)等免疫相关基因在幼鱼鳃、肝脏、肠道和脾脏中的转录水平表达量。结果显示:(1)在幼鱼的鳃中,TNFα 和 IL-1R2 基因转录水平表达量在胁迫1 d和14 d时极显著低于对照组(p<0.01),胁迫 28 d则分别表现为极显著(p<0.01)和显著下降(p<0.05);TNFAIP3在胁迫1 d时极显著升高(p<0.01),在胁迫7 d和14 d时则分别表现为显著(p<0.05)和极显著(p<0.01)下降;IL-1β在胁迫1 d时极显著下降(p<0.01)后极显著升高 (p<0.01);IL-17C在胁迫7 d和14 d时极显著下降(p<0.01);HSP70 在胁迫1 d时显著下降(p<0.05),在胁迫14 d和28 d时均极显著降低(p<0.01);(2)在肝脏中,TNFα 和 IL-1R2 基因转录水平表达量在胁迫1 d和28 d时显著下降(p<0.05),在胁迫14 d时则极显著降低(p<0.01);TNFAIP3在胁迫1 d时极显著上升(p<0.01),在胁迫14 d和28 d时则极显著下降(p<0.01);IL-1β在所有胁迫时间点均极显著上升(p<0.01);IL-17C 在胁迫7 d、14 d和28 d时均极显著低于对照水平(p<0.01);HSP70基因表达量持续上升,并于胁迫28 d时达到顶峰(p<0.01);(3)在肠道中,TNFα、IL-1β、IL-1R2、IL-17C和HSP70基因转录水平表达量在胁迫的所有时间点均极显著高于对照组(p<0.01),TNFAIP3在胁迫1 d时表达量极显著升高(p<0.01)后下降,在胁迫7 d和14 d时分别与对照组具有显著(p<0.05)和极显著差异(p<0.01);(4)在脾脏中,TNFα和IL-17C 基因转录水平表达量在胁迫1 d、7 d和14 d时极显著下降(p<0.01),在胁迫28 d时则显著降低(p<0.05);IL-1β在胁迫1 d和28 d时极显著下降(p<0.01),在胁迫7 d和14 d时则显著降低(p<0.05);IL-1R2在胁迫1 d、7 d和28 d时极显著下降(p <0.01),在胁迫14 d时则显著下降(p<0.05);HSP70基因表达量在胁迫1 d、7 d和28 d时均极显著下降(p<0.01)。研究结果表明,28 d的低氧胁迫后,军曹鱼幼鱼免疫基因的转录表达水平发生显著性变化,预示长时间的低氧可能抑制了军曹鱼的免疫功能,引发肠道炎症,并增加了军曹鱼感染病原菌的风险。Abstract: To study the effect of hypoxia on immune function of juvenile cobia (Rachycentron canadum), the transcription level of immune related genes were detected in gills, liver, intestines, and spleen after exposing to (3.15±0.21) mg/L hypoxia stress for 28 d. The results showed: (1) in gills, the transcription level of tumor necrosis factor α (TNFα) and type 2 interleukin-1 receptor (IL-1R2) extremely decreased (p<0.01) on 1 d and 14 d of stress. On 28 d of stress, TNFα extremely decreased (p<0.01) while IL-1R2 significantly decreased (p<0.05). Tumor necrosis factor alpha induced protein 3 (TNFAIP3) extremely increased (p<0.01) on 1 d and then significantly decreased (p<0.05) on 7 d and extremely decreased (p<0.01) on 14 d. Interleukin-1β (IL-1β) extremely decreased (p<0.01) on 1 d and then extremely increased (p<0.01) . Interleukin-17C (IL-17C) extremely decreased (p<0.01) on 7 d and 14 d, while heat shock protein 70 (HSP70) significantly decreased (p<0.05) on 1 d and extremely decreased (p<0.01) on 14 d and 28 d. (2) In liver, the transcription level of TNFα and IL-1R2 significantly decreased (p<0.05) on 1 d and 28 d and extremely decreased (p<0.01) on 14 d. TNFAIP3 extremely increased (p<0.01) on 1 d and extremely decreased (p<0.01) on 14 d and 28 d. IL-1β extremely increased (p<0.01) at all stress time points, while IL-17C extremely decreased (p<0.01) on 7 d, 14 d and 28 d. HSP70 continued to increase and reached the maximum on 28 d (p<0.01). (3) In the intestine, the transcription level of TNFα, IL-1β, IL-1R2, IL-17C and HSP70 genes were higher (p<0.01) than those in the control group at all stress time points. TNFAIP3 extremely increased (p<0.01) on 1 d and significantly decreased (p<0.05) on 7 d and extremely decreased (p<0.01) on 14 d. (4) In spleen, the transcription level of TNFα and IL-17C extremely decreased (p<0.01) on 1 d, 7 d, 14 d and significantly decreased (p<0.05) on 28 d. IL-1β extremely decreased (p<0.01) on 1 d, 28 d and significantly decreased (p<0.05) on 7 d and 14 d. IL-1R2 extremely decreased (p<0.01) on 1 d, 7 d, 28 d and significantly decreased (p<0.05) on 14 d, while HSP70 extremely decreased (p<0.01) on 1 d, 7 d and 28 d. The results indicated that immune genes of transcription level in juvenile cobia showed significantly change after 28 d of hypoxia stress, suggesting that long-term hypoxia may inhibit the immune function, cause intestinal inflammation, and increase the risk of pathogen infection in cobia.
-
Key words:
- Rachycentron canadum /
- hypoxia /
- immune gene
-
图 1 低氧胁迫后军曹鱼幼鱼鳃组织TNFα(a)、TNFAIP3(b)、IL-1β(c)、IL-1R2(d)、IL-17C(e)和HSP70(f)基因的相对表达量
*表示显著差异(p<0.05),**表示极显著差异(p<0.01)
Fig. 1 Relative expression of TNFα (a), TNFAIP3 (b), IL-1β (c), IL-1R2 (d), IL-17C (e) and HSP70 (f) genes in gill of juvenile cobia after hypoxia
*Shows significant difference (p<0.05), **shows extremely significant difference (p<0.01)
图 2 低氧胁迫后军曹鱼幼鱼肝组织TNFα(a)、TNFAIP3(b)、IL-1β(c)、IL-1R2(d)、IL-17C(e)和HSP70(f)基因的相对表达量
*表示显著差异(p<0.05),**表示极显著差异(p<0.01)
Fig. 2 Relative expression of TNFα (a), TNFAIP3 (b), IL-1β (c), IL-1R2 (d), IL-17C (e) and HSP70 (f) genes in liver of juvenile cobia after hypoxia
*Shows significant difference (p<0.05), **shows extremely significant difference (p<0.01)
图 3 低氧胁迫后军曹鱼幼鱼肠组织TNFα(a)、TNFAIP3(b)、IL-1β(c)、IL-1R2(d)、IL-17C(e)和HSP70(f)基因的相对表达量
*表示显著差异(p<0.05),**表示极显著差异(p<0.01)
Fig. 3 Relative expression of TNFα (a), TNFAIP3 (b), IL-1β (c), IL-1R2 (d), IL-17C (e) and HSP70 (f) genes in intestine of juvenile cobia after hypoxia
*Shows significant difference (p<0.05), **shows extremely significant difference (p<0.01)
图 4 低氧胁迫后军曹鱼幼鱼脾组织TNFα(a)、TNFAIP3(b)、IL-1β(c)、IL-1R2(d)、IL-17C(e)和HSP70(f)基因的相对表达量
*表示显著差异(p<0.05),**表示极显著差异(p<0.01)
Fig. 4 Relative expression of TNFα (a), TNFAIP3 (b), IL-1β (c), IL-1R2 (d), IL-17C (e) and HSP70 (f) genes in spleen of juvenile cobia after hypoxia
*Shows significant difference (p<0.05), **shows extremely significant difference (p<0.01)
表 1 本研究所用引物序列
Tab. 1 Primer sequences used in this study
引物名称 序列(5’−3’) 用途 TNFα-F GCTGGAGTGGAAGAACGGTCAA qRT-PCR TNFα-R CTTGCCGCCTATGGAGTCTGAG qRT-PCR TNFAIP3-F TCCAGAATGCCTCACGGTGTC qRT-PCR TNFAIP3-R AGTGTGGTGTGCGATGTGTCA qRT-PCR IL-1β-F GCAGAGAATCGGCACAGACA qRT-PCR IL-1β-R GAAGGTTCGGTAGCGGTTGT qRT-PCR IL-1R2-F ACCAAGGAGGAAGACACGAAGT qRT-PCR IL-1R2-R TGCCGAGCCAGGATGTAATCA qRT-PCR IL-17C-F GCCAAAGAAATGCGAGGAGATG qRT-PCR IL-17C-R ACAGAGGCACTCAGCGAAGA qRT-PCR HSP70-F TCACTGGAGTCCTACGCCTAC qRT-PCR HSP70-R GCTGATGCTCATACTCGTCCTT qRT-PCR β-actin-F AGGGAAATTGTGCGTGAC qRT-PCR β-asctin-R AGGCAGCTCGTAGCTCTT qRT-PCR -
[1] Mogensen T H. Pathogen recognition and inflammatory signaling in innate immune defenses[J]. Clinical Microbiology Reviews, 2009, 22(2): 240−273. doi: 10.1128/CMR.00046-08 [2] 甘桢, 王蓓, 鲁义善, 等. 罗非鱼免疫学研究进展[J]. 生物技术通报, 2014(11): 32−39.Gan Zhen, Wang Bei, Lu Yishan, et al. Research progress on tilapia immunology[J]. Biotechnology Bulletin, 2014(11): 32−39. [3] 周光炎. 免疫学原理[M]. 3版. 北京: 科学出版社, 2013.Zhou Guangyan. Principles of Immunology[M]. 3rd ed. Beijing: Science Press, 2013. [4] Press C M L, Evensen Ø. The morphology of the immune system in teleost fishes[J]. Fish & Shellfish Immunology, 1999, 9(4): 309−318. [5] Salinas I. The mucosal immune system of teleost fish[J]. Biology, 2015, 4(3): 525−539. doi: 10.3390/biology4030525 [6] Rombout J H W M, Abelli L, Picchietti S, et al. Teleost intestinal immunology[J]. Fish & Shellfish Immunology, 2011, 31(5): 616−626. [7] Parker G A, Picut C A. Liver immunobiology[J]. Toxicologic Pathology, 2005, 33(1): 52−62. doi: 10.1080/01926230590522365 [8] Zapata A, Diez B, Cejalvo T, et al. Ontogeny of the immune system of fish[J]. Fish & Shellfish Immunology, 2006, 20(2): 126−136. [9] Secombes C J, Hardie L J, Daniels G. Cytokines in fish: an update[J]. Fish & Shellfish Immunology, 1996, 6(4): 291−304. [10] Nur I, Abdelkhalek N K, Motobe S, et al. Functional analysis of membrane-bound complement regulatory protein on T-cell immune response in ginbuna crucian carp[J]. Molecular Immunology, 2016, 70: 1−7. doi: 10.1016/j.molimm.2015.11.010 [11] Wang Rujia, Feng Jianbin, Li Chao, et al. Four lysozymes (one c-type and three g-type) in catfish are drastically but differentially induced after bacterial infection[J]. Fish & Shellfish Immunology, 2013, 35(1): 136−145. [12] Nam S E, Haque M N, Shin Y K, et al. Constant and intermittent hypoxia modulates immunity, oxidative status, and blood components of red seabream and increases its susceptibility to the acute toxicity of red tide dinoflagellate[J]. Fish & Shellfish Immunology, 2020, 105: 286−296. [13] Abdel-Tawwab M, Hagras A E, Elbaghdady H A M, et al. Effects of dissolved oxygen and fish size on Nile tilapia, Oreochromis niloticus (L.): growth performance, whole-body composition, and innate immunity[J]. Aquaculture International, 2015, 23(5): 1261−1274. doi: 10.1007/s10499-015-9882-y [14] Kvamme B O, Gadan K, Finne-Fridell F, et al. Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress[J]. Fish & Shellfish Immunology, 2013, 34(1): 55−65. [15] Ortuño J, Esteban M A, Meseguer J. Lack of effect of combining different stressors on innate immune responses of seabream (Sparus aurata L.)[J]. Veterinary Immunology and Immunopathology, 2002, 84(1/2): 17−27. [16] Molina W F, Benetti D D, Fiorentino J N, et al. Early sex shape dimorphism (SShD) in Rachycentron canadum (Linnaeus, 1766) and its applications for monosex culture[J]. Aquaculture, 2018, 495: 320−327. doi: 10.1016/j.aquaculture.2018.05.056 [17] Food and Agriculture Organization of the United Nations. The state of world fisheries and aquaculture 2020[R/OL]. [2021−01−15]. https://creativecommons.org/licenses/by-nc-sa/3.0/igo [18] Han Yingli, Hou Congcong, Du Chen, et al. Molecular cloning and expression analysis of five heat shock protein 70 (HSP70) family members in Lateolabrax maculatus with Vibrio harveyi infection[J]. Fish & Shellfish Immunology, 2017, 60: 299−310. [19] Kong Yidi, Gao Chunshan, Du Xiaoyan, et al. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus)[J]. Fish & Shellfish Immunology, 2020, 102: 412−421. [20] Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho J K. Role of cytokines in inflammatory bowel disease[J]. World Journal of Gastroenterology, 2008, 14(27): 4280−4288. doi: 10.3748/wjg.14.4280 [21] Mérour E, Jami R, Lamoureux A, et al. A20 (tnfaip3) is a negative feedback regulator of RIG-I-Mediated IFN induction in teleost[J]. Fish & Shellfish Immunology, 2019, 84: 857−864. [22] An Feimeng, Wang Jiaqi, Gao Hongyan, et al. Impact of IL1R1 and IL1R2 gene polymorphisms on risk of osteonecrosis of the femoral head from a case-control study[J]. Molecular Genetics & Genomic Medicine, 2019, 7(3): e00557. [23] Ding Yang, Ao Jingqun, Chen Xinhua. Comparative study of interleukin-17C (IL-17C) and IL-17D in large yellow croaker Larimichthys crocea reveals their similar but differential functional activity[J]. Developmental & Comparative Immunology, 2017, 76: 34−44. [24] Song Linsheng, Wu Longtao, Ni Duojiao, et al. The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress[J]. Fish & Shellfish Immunology, 2006, 21(4): 335−345. [25] Mu Weijie, Wen Haishen, Li Jifang, et al. Cloning and expression analysis of a HSP70 gene from Korean rockfish (Sebastes schlegeli)[J]. Fish & Shellfish Immunology, 2013, 35(4): 1111−1121. [26] 矫婉莹. 镉和毒死蜱暴露导致鲤鱼鳃免疫损伤机理的研究[D]. 哈尔滨: 东北农业大学, 2019.Jiao Wanying. The study on the mechanism of immune injury induced by cadmium and chlorpyrifos exposure in common carp gills[D]. Harbin: Northeast Agricultural University, 2019. [27] 董忠典, 黎学友, 廖健, 等. 雌、雄弓背青鳉(Oryzias curvinotus)肝脏转录组比较分析[J]. 海洋与湖沼, 2020, 51(5): 1203−1213.Dong Zhongdian, Li Xueyou, Liao Jian, et al. Comparative transcriptome analysis of male and female liver of Oryzias curvinotus[J]. Oceanologia et Limnologia Sinica, 2020, 51(5): 1203−1213. [28] Freitas-Lopes M A, Mafra K, David B A, et al. Differential location and distribution of hepatic immune cells[J]. Cells, 2017, 6(4): 48. doi: 10.3390/cells6040048 [29] 王维政, 曾泽乾, 黄建盛, 等. 低氧胁迫对军曹鱼幼鱼生长、血清生化和非特异性免疫指标的影响[J]. 海洋学报, 2021, 43(2): 49−58.Wang Weizheng, Zeng Zeqian, Huang Jiansheng, et al. Hypoxia stress on growth, serum biochemical and non-specific immune indexes of juvenile cobia (Rachycentron canadum)[J]. Haiyang Xuebao, 2021, 43(2): 49−58. [30] 陈世喜, 王鹏飞, 区又君, 等. 急性和慢性低氧胁迫对卵形鲳鲹鳃器官的影响[J]. 南方水产科学, 2017, 13(1): 124−130. doi: 10.3969/j.issn.2095-0780.2017.01.016Chen Shixi, Wang Pengfei, Ou Youjun, et al. Acute and chronic hypoxia effect on gills of golden pompano (Trachinotus ovatus)[J]. South China Fisheries Science, 2017, 13(1): 124−130. doi: 10.3969/j.issn.2095-0780.2017.01.016 [31] 陈世喜, 王鹏飞, 区又君, 等. 急性和慢性低氧胁迫对卵形鲳鲹幼鱼肝组织损伤和抗氧化的影响[J]. 动物学杂志, 2016, 51(6): 1049−1058.Chen Shixi, Wang Pengfei, Ou Youjun, et al. The effect of acute and chronic hypoxia stress on liver tissue structure and oxidation in juvenile golden pompano (Trachinotus ovatus)[J]. Chinese Journal of Zoology, 2016, 51(6): 1049−1058. [32] Zhang Xiaoyan, Wen Haishen, Wang Hailiang, et al. RNA-Seq analysis of salinity stress–responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus)[J]. PLoS One, 2017, 12(3): e0173238. doi: 10.1371/journal.pone.0173238 [33] Parra D, Korytář T, Takizawa F, et al. B cells and their role in the teleost gut[J]. Developmental & Comparative Immunology, 2016, 64: 150−166. [34] Sundh H, Kvamme B O, Fridell F, et al. Intestinal barrier function of Atlantic salmon (Salmo salar L.) post smolts is reduced by common sea cage environments and suggested as a possible physiological welfare indicator[J]. BMC Physiology, 2010, 10: 22. doi: 10.1186/1472-6793-10-22 [35] Xavier R J, Podolsky D K. Unravelling the pathogenesis of inflammatory bowel disease[J]. Nature, 2007, 448(7152): 427−434. doi: 10.1038/nature06005 [36] Maloy K J, Antonelli L R V, Lefevre M, et al. Cure of innate intestinal immune pathology by CD4+ CD25+ regulatory T cells[J]. Immunology Letters, 2005, 97(2): 189−192. doi: 10.1016/j.imlet.2005.01.004 [37] Wang Weizheng, Huang Jiansheng, Zhang Jiandong, et al. Effects of hypoxia stress on the intestinal microflora of juvenile of cobia (Rachycentron canadum)[J]. Aquaculture, 2021, 536: 736419. doi: 10.1016/j.aquaculture.2021.736419 [38] Niklasson L, Sundh H, Fridell F, et al. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions[J]. Fish & Shellfish Immunology, 2011, 31(6): 1072−1080. [39] Lieschke G J, Trede N S. Fish immunology[J]. Current Biology, 2009, 19(16): R678−R682. doi: 10.1016/j.cub.2009.06.068 [40] Wluka A, Olszewski W L. Innate and adaptive processes in the spleen[J]. Annals of Transplantation, 2006, 11(4): 22−29. [41] Mu Yinnan, Li Wanru, Wu Bin, et al. Transcriptome analysis reveals new insights into immune response to hypoxia challenge of large yellow croaker (Larimichthys crocea)[J]. Fish & Shellfish Immunology, 2020, 98: 738−747. -





 下载:
下载: