Sequential extraction procedure and element occurrence states of hydrothermal sediments from the South Atlantic Ridge
-
摘要: 本文对采自南大西洋受不同程度热液活动影响的表层沉积物样品进行了元素和矿物组成分析,并对热液沉积物的碳酸盐相、Fe-Mn氧化物相和残渣态进行了一系列顺序提取实验。选用不同浓度的盐酸羟胺(HH)和醋酸(HAc)混合溶液对样品的Fe-Mn氧化物相进行提取,通过分析不同实验条件下Fe-Mn氧化物相Ti/Nd、Ti/Pb比值和Fe-Mn氧化物相、残渣态的稀土元素(REE)标准化配分模式及Ce和Eu异常值,确定了不同类型热液沉积物样品Fe-Mn氧化物相提取的理想试剂条件均为0.5 mol/L HH和25% HAc混合溶液。研究结果表明,受热液活动影响程度越高,沉积物中Fe、Cu、Zn等元素含量越高,Ca、Sr、Ba含量呈相反趋势,Mn、Pb和REE未受到热液活动明显影响;随着受热液活动影响增强,Ca、Sr、Nd在Fe-Mn氧化物相中所占比例增加,在残渣态中降低,Mn、Co、Ni和Zn呈相反趋势,Cu在碳酸盐相所占比例增加,在残渣态中降低,Pb赋存状态不受热液活动影响,主要赋存于Fe-Mn氧化物相;REE主要赋存于残渣态,沉积物受热液活动影响越明显,残留相对REE富集能力越强,残渣态REE球粒陨石标准化配分模式表现为LREE相对富集越来越不明显的特征。本文研究为进一步了解南大西洋热液沉积物特征和热液活动对沉积物元素赋存状态影响提供了方法和地球化学数据支持。Abstract: In this study, elemental and mineral compositions of three surface sediments collected from the South Atlantic Ridge affected by hydrothermal activities of various degrees were analyzed, and series of extraction experiments for carbonate phase, Fe-Mn oxide phase and insoluble residual phase were carried out. Mixed reagent of hydroxylamine hydrochloride (HH) with different concentrations and 25% acetic acid (HAc) were used to extract Fe-Mn oxide phase from the sample. In order to corroborate the reliability of the extracting methods, Ti/Nd and Ti/Pb ratios of the Fe-Mn oxide phase, rare earth elements (REE) patterns as well as δCe and δEu ratios of different chemical phase were used to determine that the ideal reagent conditions for extracting Fe-Mn oxide phase from three different types of hydrothermal sediments were all 0.5 mol/L HH in 25% acetic acid. The results show that the higher the degree of influence of hydrothermal activities, the higher the contents of Fe, Cu, Zn and other elements in the sediments, and the contents of Ca, Sr and Ba show an opposite trend. Manganese, Pb and REE are not significantly affected by hydrothermal activities. As the influence of hydrothermal activity increases, the proportion of Ca, Sr and Nd increases in the Fe-Mn oxide phase and decreases in the residual phase, while Mn, Co, Ni and Zn have an opposite trend, and the proportion of Cu increases in the carbonate phase and decreases in the residual phase. Lead is not affected by the influence strength of hydrothermal activity and mainly occurs in the Fe-Mn oxide phase. REE mainly occur in the residue state. The occurrence state of REE shows that the more significant influence by the hydrothermal activities of the sediments, the more enrichment of REE in the residual phase. And the chondrite-normalized REE patterns of the residual phase exhibit that the enrichment of light REE are less obvious. This study provides methods for extracting hydrothermal fractions and valuable geochemical data for further understanding of the characteristics of hydrothermal sediments and the effects of hydrothermal activities on the occurrence state of elements in the South Atlantic Ridge.
-
表 1 样品全岩及非碳酸盐组分化学分析结果
Tab. 1 The chemical composition of three sediment samples and the carbonate-free fraction
样品号 Al2O3 CaO Fe2O3 K2O Na2O MgO MnO Ti Al/Ti Ba Sr Co Ni Cu Zn Cr V Mo 全样 33II-12 1.00 48.33 1.94 0.31 1.78 0.75 0.18 446 42.3 162 1620 15 19 69 22 6.35 38 1.62 26V-04 3.49 41.31 4.17 0.30 2.31 1.91 0.17 2092 31.5 300 1734 23 27 416 112 37 83 1.16 26III-04 13.33 0.84 25.80 0.21 1.32 15.43 0.21 5071 49.7 9.67 52 110 84 10012 976 163 433 16.12 非碳酸盐 33II-12 11.99 2.35 23.36 1.56 1.00 3.70 1.92 4826 − 1765 143 150 140 791 233 84 612 15.72 26V-04 13.23 7.66 17.39 0.48 1.84 5.59 0.69 8514 − 1175 181 86 100 1805 314 166 472 4.42 26III-04 10.33 0.50 25.16 0.21 0.31 13.16 0.21 5340 − 1.98 10 100 83 5259 944 189 581 13.10 样品号 Pb Th U La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Y 全样 33II-12 11.85 1.15 0.32 11.85 14.10 2.75 11.06 2.43 0.60 2.42 0.41 2.63 0.50 1.38 0.21 1.19 0.18 15.90 26V-04 23.93 0.90 0.36 10.55 13.63 2.61 11.19 2.62 0.77 2.69 0.48 3.03 0.59 1.74 0.26 1.54 0.24 18.13 26III-04 12.57 0.55 4.79 5.33 10.68 1.92 9.22 2.90 0.79 3.32 0.67 4.67 0.91 2.81 0.41 2.49 0.42 25.01 非碳酸盐 33II-12 145 10.96 1.31 36.31 169 8.68 34.23 6.99 1.99 7.15 1.23 7.87 1.53 4.41 0.69 4.26 0.66 44.15 26V-04 107 2.67 0.62 14.64 48.24 4.14 18.03 4.57 1.59 4.96 0.96 6.32 1.27 3.63 0.58 3.54 0.56 32.45 26III-04 12.91 0.53 4.42 4.56 9.82 1.66 8.16 2.59 0.67 2.84 0.64 4.45 0.91 2.60 0.41 2.55 0.42 23.93 注:氧化物单位为%,其他元素单位为μg/g。 表 2 不同实验条件下元素回收率(%)
Tab. 2 The recovery rate of elements under different experimental conditions (%)
样品号 C(HH)/(mol·L−1) Al Ca Fe Mg Mn Ti Sr Co Ni Cu Zn Cr Pb Nd 26V-04 2 94.7 112.1 92.0 94.7 90.1 95.7 93.7 83.3 122.9 96.7 90.0 122.6 100.8 87.1 1 93.1 111.9 88.4 93.3 91.4 89.9 93.6 86.1 122.2 96.5 87.2 123.0 97.7 86.2 0.5 89.4 111.4 88.4 90.5 88.5 88.3 93.5 82.1 119.3 96.1 89.4 112.3 98.3 85.5 0.25 95.3 112.0 93.2 98.0 92.6 97.1 93.6 87.7 129.3 100.0 91.8 124.6 90.6 88.8 0.1 91.3 111.4 97.6 88.6 91.1 94.4 93.8 84.1 121.0 111.9 97.9 116.1 121.1 91.2 26III-04 2 102.1 107.0 98.4 100.5 95.8 97.3 98.2 94.6 97.9 92.9 97.9 116.7 92.5 91.5 1 88.5 98.3 98.2 92.0 95.4 97.6 93.9 92.6 100.0 92.2 97.9 121.1 91.7 92.9 0.5 95.2 97.4 98.0 95.4 95.7 97.3 93.7 91.5 97.4 93.5 98.4 116.7 91.9 94.4 0.25 101.8 93.0 97.5 102.2 94.5 97.5 91.7 92.7 99.5 92.8 98.6 123.6 90.0 94.3 0.1 104.0 92.3 101.5 101.3 96.7 100.5 93.0 92.9 104.9 95.4 102.4 119.6 100.0 94.0 表 3 不同实验条件下提取出的沉积物各相态百分比(%)
Tab. 3 Proportion of main phases from experimental investigations (%)
C(HH)/(mol·L−1) 样品33II-12 样品26V-04 样品26III-04 碳酸盐相 Fe-Mn氧化物相 残渣态 碳酸盐相 Fe-Mn氧化物相 残渣态 碳酸盐相 Fe-Mn氧化物相 残渣态 2 92.57 1.33 6.10 76.71 3.05 20.24 5.14 10.86 84.00 1 92.54 1.07 6.38 78.10 2.79 19.11 5.54 9.32 85.14 0.5 92.58 0.90 6.52 78.23 2.56 19.20 5.46 7.92 86.62 0.25 92.95 0.69 6.37 77.37 1.99 20.64 5.38 6.59 88.02 0.1 − − − 77.11 2.09 20.80 5.23 5.10 89.67 注:−表示无数据。 表 4 不同实验条件下代表性元素在各相态中百分比(%)
Tab. 4 Percentage of representative elements in different chemical phase from leaching tests (%)
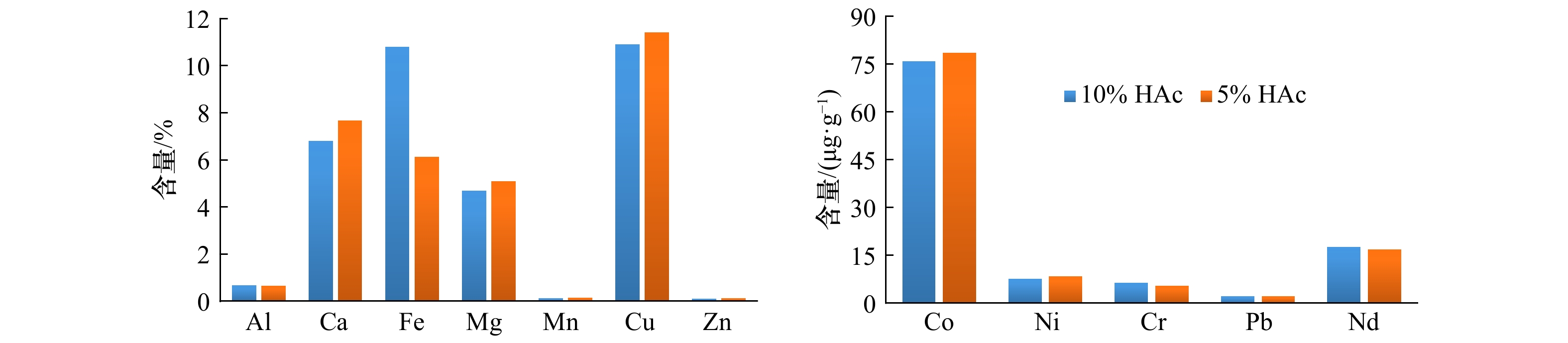
样品号 相态 C(HH)/(mol·L−1) Al2O3 CaO Fe2O3 MgO MnO Ti Sr Co Ni Cu Zn Cr Pb Nd 33II-12 碳酸盐相 4.67 99.61 2.53 56.06 13.35 0.28 99.07 0.00 56.99 30.41 26.19 4.74 2.02 73.61 Fe-Mn氧化物相 2 4.21 0.12 30.66 4.91 82.65 2.18 0.30 90.96 23.63 26.01 24.87 11.09 90.69 15.68 1 3.98 0.13 27.64 4.59 83.68 1.41 0.35 90.07 26.02 24.35 25.67 8.42 84.21 16.47 0.5 3.13 0.12 22.97 3.89 83.74 0.96 0.32 89.25 23.96 19.98 22.02 7.55 68.25 13.62 0.25 2.68 0.11 19.84 3.32 82.71 0.47 0.30 88.01 22.90 17.92 19.89 6.38 47.78 11.57 残渣态 2 90.91 0.21 66.69 36.33 2.65 97.53 0.56 9.04 14.13 41.69 47.69 82.95 7.24 8.72 1 91.36 0.27 69.83 39.34 2.97 98.31 0.58 9.93 16.99 45.24 48.14 86.84 13.77 9.92 0.5 92.15 0.24 74.56 38.74 3.41 98.76 0.58 10.75 17.37 50.43 52.43 87.20 29.82 11.93 0.25 92.43 0.26 77.60 39.37 4.58 99.24 0.56 11.99 18.16 50.76 53.77 88.31 50.24 13.69 26V-04 碳酸盐相 4.49 95.80 2.04 27.45 8.89 0.09 97.38 0.00 30.36 28.80 39.50 6.08 1.51 60.67 Fe-Mn氧化物相 2 5.75 0.21 39.15 6.72 73.26 2.70 0.83 63.68 21.83 40.33 28.93 9.87 90.86 19.08 1 5.32 0.21 36.78 5.44 74.10 1.49 0.86 64.37 20.32 39.47 28.25 7.70 84.61 18.83 0.5 4.58 0.18 32.69 3.67 73.95 0.79 0.81 65.57 18.01 34.29 25.90 6.33 65.32 16.35 0.25 4.07 0.16 29.85 3.14 72.32 0.36 0.78 61.64 16.48 28.79 24.04 5.12 51.39 14.83 0.1 3.96 0.18 26.40 3.65 73.18 0.21 0.82 64.03 16.02 20.75 21.71 5.03 23.48 13.02 残渣态 2 89.76 3.99 58.81 65.83 17.85 97.20 1.79 36.32 47.80 30.87 31.58 84.05 7.64 20.25 1 90.11 3.81 61.10 66.70 17.13 98.41 1.67 35.63 49.13 31.66 30.98 86.23 13.83 19.87 0.5 90.67 3.45 65.18 67.60 17.00 99.11 1.68 34.43 50.71 36.71 34.31 87.02 33.13 21.83 0.25 91.47 3.98 68.13 70.33 19.03 99.55 1.78 38.36 54.67 43.33 37.24 88.89 46.94 25.63 0.1 91.39 3.40 71.68 67.00 18.02 99.70 1.89 35.97 53.15 54.36 41.95 88.55 75.26 29.04 26III-04 碳酸盐相 0.25 34.76 1.87 1.30 2.49 0.00 58.50 3.56 0.55 56.66 4.39 0.19 0.99 10.29 Fe-Mn氧化物相 2 3.13 22.60 26.96 3.07 25.97 0.51 23.98 30.41 5.09 30.57 21.60 5.21 86.90 50.91 1 2.57 16.22 21.73 2.66 24.51 0.22 19.94 29.72 4.15 27.77 15.23 3.59 80.86 47.42 0.5 1.81 12.92 17.23 2.01 23.71 0.10 18.02 25.61 3.12 25.47 11.28 2.82 68.97 41.70 0.25 1.25 11.32 14.36 1.53 22.85 0.04 16.46 24.80 2.43 22.72 8.51 2.02 56.00 35.10 0.1 0.91 10.22 13.57 1.46 22.92 0.02 16.21 24.73 2.43 22.47 7.33 2.04 42.27 31.73 残渣态 2 96.62 42.64 71.17 95.63 71.53 99.49 17.52 66.03 94.36 12.77 74.01 94.60 12.11 38.80 1 97.14 45.95 76.39 95.93 72.99 99.77 18.90 66.64 95.32 15.11 80.38 96.22 18.14 42.44 0.5 97.92 48.90 80.89 96.62 73.80 99.90 20.69 70.71 96.33 18.22 84.35 96.99 30.04 48.32 0.25 98.49 48.68 83.76 97.20 74.62 99.96 20.90 71.57 97.03 20.54 87.13 97.80 42.98 54.91 0.1 98.84 49.47 84.61 97.25 74.61 99.98 22.01 71.64 97.05 22.34 88.48 97.78 56.81 58.24 表 5 不同实验条件下各相态中元素含量和元素比
Tab. 5 Element concentrations and element ratios in different chemical phase from leaching tests
样品号 相态 C(HH)/(mol·L−1) Al2O3 CaO Fe2O3 MgO MnO Ti Sr Co Ni Cu Zn Cr Pb Nd Al/Ti Ti/Pb Ti/Nd 33II-12 碳酸盐相 0.05 58.15 0.05 0.40 0.02 1.13 1450 0.00 17 22 6 0.39 0.23 7.52 228 4.95 0.15 Fe-Mn氧化物相 2 3.88 6.27 50.78 3.09 10.99 787 404 835 589 1 589 521 67 921 143 26 0.85 5.48 1 3.58 6.33 44.97 2.84 11.48 500 440 907 664 1 525 549 61 822 145 38 0.61 3.45 0.5 3.32 6.95 45.73 2.81 14.25 396 476 1076 709 1 537 577 58 833 142 44 0.48 2.79 0.25 3.59 8.44 49.69 3.13 18.57 244 589 1393 880 1 698 659 64 740 157 78 0.33 1.56 残渣态 2 13.79 1.88 18.20 3.78 0.06 5796 125 13.67 58 433 171 83 12 13 12.59 478 440 1 13.82 2.28 19.10 4.09 0.07 5851 124 16.82 73 476 173 105 23 15 12.50 259 398 0.5 13.49 1.99 20.45 3.86 0.08 5632 121 17.84 71 534 189 93 50 17 12.68 112 329 0.25 13.40 2.21 21.02 4.02 0.11 5625 120 20.52 75 520 193 96 84 20 12.61 67 281 26V-04 碳酸盐相 0.19 57.34 0.10 0.64 0.02 2.42 2045 0.00 13 150 51 3.54 0.47 7.65 419 5.15 0.32 Fe-Mn氧化物相 2 6.23 3.26 49.25 3.99 3.74 1775 444 393 240 5 328 956 146 720 61 19 2.47 29.07 1 6.19 3.40 48.59 3.47 4.19 1007 500 449 243 5 687 989 125 710 65 33 1.42 15.45 0.5 5.56 3.14 47.00 2.47 4.41 567 513 474 229 5 350 1010 102 600 61 52 0.94 9.29 0.25 6.80 3.72 58.34 2.96 5.82 369 640 614 293 6 029 1244 118 561 74 98 0.66 4.97 0.1 6.02 3.87 51.31 2.95 5.50 197 641 580 253 4 618 1136 102 325 64 162 0.61 3.10 残渣态 2 14.65 9.12 11.14 5.88 0.14 9613 144 33.71 79 614 157 187 9 9.75 8.07 1056 985 1 15.30 9.23 11.78 6.22 0.14 9692 142 36.22 86 666 158 203 17 10.04 8.36 572 966 0.5 14.71 8.28 12.51 6.08 0.14 9535 142 33.22 86 765 179 187 41 10.88 8.17 235 877 0.25 14.72 8.91 12.81 6.37 0.15 9796 140 36.77 93 873 185 197 49 12.34 7.95 199 794 0.1 13.99 7.53 14.02 5.45 0.14 9463 148 32.81 84 1 217 221 181 105 14.26 7.83 90 664 26III-04 碳酸盐相 0.66 5.97 9.09 3.84 0.10 2.45 568 70.79 8.67 10 0847 802 6.91 2.20 16.61 1417 1.12 0.15 Fe-Mn氧化物相 2 3.92 1.87 62.98 4.38 0.49 231 112 291 38.58 26 192 1899 91 93 39.55 90 2.48 5.84 1 3.25 1.43 59.04 4.04 0.53 118 104 324 37.43 27 490 1560 76 100 43.53 145 1.18 2.72 0.5 2.89 1.33 55.05 3.74 0.61 63 110 325 32.29 30 133 1368 68 101 45.83 244 0.62 1.37 0.25 2.57 1.34 54.78 3.65 0.70 29 118 383 30.84 32 031 1242 62 96 46.29 477 0.30 0.62 0.1 2.48 1.55 69.64 4.48 0.92 19 153 495 42.03 42 069 1433 78 104 53.84 683 0.18 0.36 残渣态 2 15.65 0.46 21.50 17.65 0.17 5843 10.60 81.72 92.53 1 414 841 214 1.68 3.90 14.18 3485 1499 1 13.46 0.45 22.72 15.99 0.17 5797 10.79 79.57 94.22 1 638 902 223 2.46 4.27 12.29 2360 1359 0.5 14.34 0.46 23.61 16.41 0.17 5692 11.58 82.07 91.17 1 970 935 213 4.01 4.85 13.33 1421 1173 0.25 15.17 0.43 23.94 17.41 0.17 5616 11.27 82.81 92.24 2 169 952 224 5.53 5.42 14.30 1016 1036 0.1 15.28 0.43 24.71 16.96 0.17 5683 11.81 81.57 95.49 2 380 985 213 7.96 5.62 14.23 714 1010 注:氧化物单位为%,其他元素单位为μg/g。 表 6 不同实验条件下各相态稀土元素δCe和δEu值
Tab. 6 δCe ratios and δEu ratios of different chemical phase from leaching tests in hydrothermal sediments on the South Atlantic
C(HH)/(mol·L−1) 33II-12 26V-04 26III-04 Fe-Mn氧化物相 残渣态 Fe-Mn氧化物相 残渣态 Fe-Mn氧化物相 残渣态 δCe δEu δCe δEu δCe δEu δCe δEu δCe δEu δCe δEu 2 2.02 0.68 1.12 1.26 1.75 0.73 1.14 1.13 0.86 1.05 0.87 0.50 1 1.82 0.64 1.22 1.13 1.64 0.68 1.22 1.09 0.86 1.01 0.84 0.51 0.5 2.00 0.66 1.40 1.03 1.60 0.71 1.34 1.26 0.87 0.98 0.87 0.58 0.25 1.98 0.68 1.45 0.88 1.57 0.73 1.28 1.16 0.86 1.02 0.86 0.57 0.1 − − − − 1.55 0.74 1.41 1.10 0.88 1.00 0.81 0.67 注:−表示无数据。 -
[1] German C R, Bennett S A, Connelly D P, et al. Hydrothermal activity on the southern Mid-Atlantic Ridge: Tectonically- and volcanically-controlled venting at 4-5°S[J]. Earth and Planetary Science Letters, 2008, 273(3/4): 332−344. [2] Haase K M, Koschinsky A, Petersen S, et al. Diking, young volcanism and diffuse hydrothermal activity on the southern Mid-Atlantic Ridge: the Lilliput field at 9°33′S[J]. Marine Geology, 2009, 266(1/4): 52−64. [3] Tao Chunhui, Li Huaiming, Yang Yaomin, et al. Two hydrothermal fields found on the Southern Mid-Atlantic Ridge[J]. Science China Earth Sciences, 2011, 54(9): 1302−1303. doi: 10.1007/s11430-011-4260-8 [4] Li Bing, Shi Xuefa, Wang Jixin, et al. Tectonic environments and local geologic controls of potential hydrothermal fields along the Southern Mid-Atlantic Ridge (12−14°S)[J]. Journal of Marine Systems, 2018, 181: 1−13. doi: 10.1016/j.jmarsys.2018.02.003 [5] Schmid F, Peters M, Walter M, et al. Physico-chemical properties of newly discovered hydrothermal plumes above the Southern Mid-Atlantic Ridge (13°−33°S)[J]. Deep−Sea Research Part I: Oceanographic Research Papers, 2019, 148: 34−52. doi: 10.1016/j.dsr.2019.04.010 [6] Li Bing, Yang Yaomin, Shi Xuefa, et al. Characteristics of a ridge-transform inside corner intersection and associated mafic-hosted seafloor hydrothermal field (14.0°S, Mid-Atlantic Ridge)[J]. Marine Geophysical Research, 2014, 35(1): 55−68. doi: 10.1007/s11001-013-9209-1 [7] 李兵, 石学法, 杨耀民, 等. 南大西洋14.0°S热液区热液硫化物矿物学特征及地质意义[J]. 矿物学报, 2015, 35(1): 35−43.Li Bing, Shi Xuefa, Yang Yaomin, et al. Mineralogy and geological significance of hydrothermal deposits from the 14.0°S hydrothermal field, South Mid-Atlantic Ridge[J]. Acta Mineralogica Sinica, 2015, 35(1): 35−43. [8] Wang Shujie, Li Huaiming, Zhai Shikui, et al. Geochemical features of sulfides from the Deyin-1 hydrothermal field at the southern Mid-Atlantic Ridge near 15°S[J]. Journal of Ocean University of China, 2017, 16(6): 1043−1054. doi: 10.1007/s11802-017-3316-6 [9] Wang Shujie, Li Huaiming, Zhai Shikui, et al. Mineralogical characteristics of polymetallic sulfides from the Deyin-1 hydrothermal field near 15°S, southern Mid-Atlantic Ridge[J]. Acta Oceanologica Sinica, 2017, 36(2): 22−34. doi: 10.1007/s13131-016-0961-3 [10] 李景喜, 朱志伟, 尹晓斐, 等. 南大西洋中脊表层沉积物中稀土元素的含量及分布模式分析[J]. 分析化学, 2015, 43(1): 21−26. doi: 10.1016/S1872-2040(15)60796-4Li Jingxi, Zhu Zhiwei, Yin Xiaofei, et al. Analysis of contents and distribution patterns of rare earth elements in surface sediments of the south Mid-Atlantic Ridge[J]. Chinese Journal of Analytical Chemistry, 2015, 43(1): 21−26. doi: 10.1016/S1872-2040(15)60796-4 [11] Xin Huang, Chen Shuai, Zeng Zhigang, et al. The influence of seafloor hydrothermal activity on major and trace elements of the sediments from the South Mid-Atlantic Ridge[J]. Journal of Ocean University of China, 2017, 16(5): 775−780. doi: 10.1007/s11802-017-3311-y [12] 刘菲菲, 于增慧, 高玉花, 等. 海洋沉积物的顺序萃取方法及其在冲绳海槽热液影响沉积物中的应用[J]. 海洋地质与第四纪地质, 2008, 28(5): 137−144.Liu Feifei, Yu Zenghui, Gao Yuhua, et al. Sequential extraction procedure for marine sediments and application to the Middle Okinawa Trough[J]. Marine Geology & Quaternary Geology, 2008, 28(5): 137−144. [13] 于增慧, 高玉花, 翟世奎, 等. 冲绳海槽中部沉积物中热液源组分的顺序淋滤萃取研究[J]. 中国科学: 地球科学, 2012, 55(4): 665−674. doi: 10.1007/s11430-011-4273-3Yu Zenghui, Gao Yuhua, Zhai Shikui, et al. Resolving the hydrothermal signature by sequential leaching studies of sediments from the middle of the Okinawa Trough[J]. Science China Earth Sciences, 2012, 55(4): 665−674. doi: 10.1007/s11430-011-4273-3 [14] Bayon G, German C R, Boella R M, et al. An improved method for extracting marine sediment fractions and its application to Sr and Nd isotopic analysis[J]. Chemical Geology, 2002, 187(3/4): 179−199. [15] Frank M. Radiogenic isotopes: tracers of past ocean circulation and erosional input[J]. Reviews of Geophysics, 2002, 40(1): 1−38. [16] Gutjahr M, Frank M, Stirling C H, et al. Reliable extraction of a deepwater trace metal isotope signal from Fe-Mn oxyhydroxide coatings of marine sediments[J]. Chemical Geology, 2007, 242(3/4): 351−370. [17] Bayon G, German C R, Burton K W, et al. Sedimentary Fe-Mn oxyhydroxides as paleoceanographic archives and the role of Aeolian flux in regulating oceanic dissolved REE[J]. Earth and Planetary Science Letters, 2004, 224(3/4): 477−492. [18] Sun Zhilei, Cao Hong, Yin Xijie, et al. Precipitation and subsequent preservation of hydrothermal Fe-Mn oxides in distal plume sediments on Juan de Fuca Ridge[J]. Journal of Marine Systems, 2018, 187: 128−140. doi: 10.1016/j.jmarsys.2018.06.014 [19] 李康, 曾志刚, 殷学博, 等. 东太平洋海隆13°N和赤道附近表层沉积物中的元素赋存状态[J]. 海洋地质与第四纪地质, 2009, 29(3): 53−60.Li Kang, Zeng Zhigang, Yin Xuebo, et al. Mode of element occurrence in surface sediments from East Pacific Rise near 13°N and the equator[J]. Marine Geology & Quaternary Geology, 2009, 29(3): 53−60. [20] 荣坤波, 曾志刚, 武力, 等. 东太平洋海隆13°N表层含金属沉积物物质组成与元素赋存状态[J]. 海洋科学, 2018, 42(7): 70−79. doi: 10.11759/hykx20170925002Rong Kunbo, Zeng Zhigang, Wu Li, et al. Composition and element occurrence states of recent metalli-ferous sediments from the East Pacific Rise at 13°N[J]. Marine Sciences, 2018, 42(7): 70−79. doi: 10.11759/hykx20170925002 [21] 张颖, 张辉, 王小静, 等. 海洋沉积物不同相态中Sr、Nd同位素提取方法研究[J]. 海洋学报, 2020, 42(2): 155−166.Zhang Ying, Zhang Hui, Wang Xiaojing, et al. Sequential extraction of Sr and Nd isotope from Fe–Mn oxyhydroxide and detrital in marine sediments[J]. Haiyang Xuebao, 2020, 42(2): 155−166. [22] Wang Hao, Li Xiaohu, Chu Fengyou, et al. Mineralogy, geochemistry, and Sr-Pb isotopic geochemistry of hydrothermal massive sulfides from the 15.2°S hydrothermal field, Mid-Atlantic Ridge[J]. Journal of Marine Systems, 2018, 180: 220−227. doi: 10.1016/j.jmarsys.2017.02.010 [23] Plank T, Langmuir C H. The chemical composition of subducting sediment and its consequences for the crust and mantle[J]. Chemical Geology, 1998, 145(3/4): 325−394. [24] 赵一阳, 翟世奎, 李永植, 等. 冲绳海槽中部热水活动的新记录[J]. 科学通报, 1997, 42(7): 574−577. doi: 10.1007/BF03182621Zhao Yiyang, Zhai Shikui, Li Yongzhi, et al. New records of submarine hydrothermal activity in middle part of the Okinawa Trough[J]. Chinese Science Bulletin, 1997, 42(7): 574−577. doi: 10.1007/BF03182621 [25] 杨宝菊, 吴永华, 刘季花, 等. 冲绳海槽表层沉积物元素地球化学及其对物源和热液活动的指示[J]. 海洋地质与第四纪地质, 2018, 38(2): 25−37.Yang Baoju, Wu Yonghua, Liu Jihua, et al. Elemental geochemistry of surface sediments in Okinawa Trough and its implications for provenance and hydrothermal activity[J]. Marine Geology & Quaternary Geology, 2018, 38(2): 25−37. [26] 翟世奎, 于增慧, 杜同军. 冲绳海槽中部现代海底热液活动在沉积物中的元素地球化学记录[J]. 海洋学报, 2007, 29(1): 58−65.Zhai Shikui, Yu Zenghui, Du Tongjun. Elemental geochemical records of modern seafloor hydrothermal activities in sediments from the central Okinawa Trough[J]. Haiyang Xuebao, 2007, 29(1): 58−65. [27] Hu Qiannan, Zhang Xin, Jiang Fuqing, et al. Geochemical characteristics of hydrothermal sediments from Iheya North Knoll in the Okinawa Trough[J]. Chinese Journal of Oceanology and Limnology, 2017, 35(4): 947−955. doi: 10.1007/s00343-017-6035-3 [28] Bloemsma M R, Zabel M, Stuut J B W, et al. Modeling the joint variability of grain size and chemical composition in sediments[J]. Sedimentary Geology, 2012, 280: 135−148. doi: 10.1016/j.sedgeo.2012.04.009 [29] Dymond J. Geochemistry of Nazca Plate surface sediments: an evalution of hydrothermal, biogenic, detrital and hydrogeneous sources[J]. Geological Society of America Memoirs, 1981, 154(12): 133−173. [30] Dunk R M, Mills R A. The impact of oxic alteration on plume-derived transition metals in ridge flank sediments from the East Pacific Rise[J]. Marine Geology, 2006, 229(3/4): 133−157. [31] Taylor S R, McLennan S M. The Continental Crust: its Composition and Evolution: an Examination of the Geochemical Record Preserved in Sedimentary Rocks[M]. Oxford: Blackwell Scientific, 1985: 312. [32] Boynton W V. Cosmochemistry of the rare earth elements: meteorite studies[J]. Developments in Geochemistry, 1984, 2: 63−114. doi: 10.1016/B978-0-444-42148-7.50008-3 [33] Mills R A, Elderfield H. Rare earth element geochemistry of hydrothermal deposits from the active TAG Mound, 26°N Mid-Atlantic Ridge[J]. Geochimica et Cosmochimica Acta, 1995, 59(17): 3511−3524. doi: 10.1016/0016-7037(95)00224-N [34] Jiang Xuejun, Lin Xuehui, Yao De, et al. Enrichment mechanisms of rare earth elements in marine hydrogenic ferromanganese crusts[J]. Science China Earth Sciences, 2011, 54(2): 197−203. doi: 10.1007/s11430-010-4070-4 [35] Piper D Z. Rare earth elements in the sedimentary cycle: a summary[J]. Chemical Geology, 1974, 14(4): 285−304. doi: 10.1016/0009-2541(74)90066-7 [36] Kuhn T, Bau M, Blum N, et al. Origin of negative Ce anomalies in mixed hydrothermal-hydrogenetic Fe–Mn crusts from the Central Indian Ridge[J]. Earth and Planetary Science Letters, 1998, 163(1/4): 207−220. [37] Elderfield H, Hawkesworth C J, Greaves M J, et al. Rare earth element geochemistry of oceanic ferromanganese nodules and associated sediments[J]. Geochimica et Cosmochimica Acta, 1981, 45(4): 513−528. doi: 10.1016/0016-7037(81)90184-8 [38] Li Zhenggang, Chu Fengyou, Jin Lu, et al. Major and trace element composition of surface sediments from the Southwest Indian Ridge: evidence for the incorporation of a hydrothermal component[J]. Acta Oceanologica Sinica, 2016, 35(2): 101−108. doi: 10.1007/s13131-015-0678-8 [39] Elderfield H, Greaves M J. Negative cerium anomalies in the rare earth element patterns of oceanic ferromanganese nodules[J]. Earth and Planetary Science Letters, 1981, 55(1): 163−170. doi: 10.1016/0012-821X(81)90095-9 [40] Klinkhammer G P, Elderfield H, Edmond J M, et al. Geochemical implications of rare earth element patterns in hydrothermal fluids from mid-ocean ridges[J]. Geochimica et Cosmochimica Acta, 1994, 58(23): 5105−5113. doi: 10.1016/0016-7037(94)90297-6 [41] 曾志刚, 翟世奎, 赵一阳, 等. 大西洋中脊TAG热液活动区中热液沉积物的稀土元素地球化学特征[J]. 海洋地质与第四纪地质, 1999, 19(3): 59−66.Zeng Zhigang, Zhai Shikui, Zhao Yiyang, et al. Rare earth element geochemistry of hydrothermal sediment from the TAG hydrothermal field, Mid-Atlantic Ridge[J]. Marine Geology & Quaternary Geology, 1999, 19(3): 59−66. [42] Murray R W, Leinen M. Scavenged excess aluminum and its relationship to bulk titanium in biogenic sediment from the central equatorial Pacific Ocean[J]. Geochimica et Cosmochimica Acta, 1996, 60(20): 3869−3878. doi: 10.1016/0016-7037(96)00236-0 [43] Kryc K A, Murray R W, Murray D W. Al-to-oxide and Ti-to-organic linkages in biogenic sediment: relationships to paleo-export production and bulk Al/Ti[J]. Earth and Planetary Science Letters, 2003, 211(1/2): 125−141. -




 下载:
下载: