Effects of Pseudoalteromonas marina flagellin on biofilm formationand settlement of Mytilus coruscus
-
摘要: 大多数海洋无脊椎动物在发育过程中都经历浮游、底栖附着阶段,厚壳贻贝(Mytilus coruscus)作为海洋经济物种与大型污损生物,其附着机制受到广泛关注。为探究海洋细菌与厚壳贻贝附着的互作关系,选取了对厚壳贻贝稚贝附着具有较高诱导活性的海洋细菌—海假交替单胞菌(Pseudoalteromonas marina),采用酸解超速离心法提取P. marina的鞭毛蛋白。将提取的鞭毛蛋白与琼脂糖溶液混合,形成凝胶直接刺激稚贝;再用提取的鞭毛蛋白处理P. marina 生物被膜进行稚贝附着实验。通过共聚焦激光扫描分析形成的生物被膜上生物量、细菌密度和胞外产物含量的变化。结果表明:P. marina 鞭毛蛋白与琼脂糖形成的混合凝胶可显著促进厚壳贻贝稚贝的附着;鞭毛蛋白处理的生物被膜对厚壳贻贝稚贝附着的诱导活性显著提高;生物被膜上的生物量、细菌密度、膜厚、胞外β-多糖、脂质和蛋白浓度都有所增加。研究表明,鞭毛蛋白可以直接调控厚壳贻贝稚贝的附着,也可通过改变P. marina 生物被膜的生物学特性,间接影响厚壳贻贝稚贝的附着,为探究细菌鞭毛蛋白与厚壳贻贝附着互作机制提供理论依据。Abstract: Most marine invertebrates undergo planktonic and benthic stages during growth. As a marine economic shellfish and macrofouling organism, Mytilus coruscus has attracted widespread attention. To explore the interaction between marine bacteria and the mussel settlement, Pseudoalteromonas marina, which has a high inducing activity for the plantigrade settlement of the M. coruscus was chosen and the flagellin was extracted by acid hydrolysis ultracentrifugation. The impact of extracted flagellin mixed with agarose solution to on plantigrade settlement was tested directly. The P. marina biofilms treated with extracted flagellin were used to investigate the inducing capacity on plantigrade settlement. Changes in biomass, bacterial density and extracellular products of the biofilms were analyzed by confocal laser scanning microscopy. The results showed that the mixed gel containing P. marina flagellin and agarose significantly promoted the settlement of plantigrades. In other treatment, the flagellin-treated biofilms also had a high inductivity of plantigrade settlement. Biomass, bacterial density, membrane thickness, extracellular β-polysaccharide, lipid and protein contents all increased. The study reveals that flagellin can directly regulate the settlement of plantigrades and control the inductivity by changing the biological characteristics of the P. marina biofilm indirectly. The study is valuable for clarifying the interaction between bacterial flagellin and plantigrade settlement of the M. coruscus.
-
Key words:
- biofilm /
- flagellin /
- Mytilus coruscus /
- plantigrade /
- settlement
-
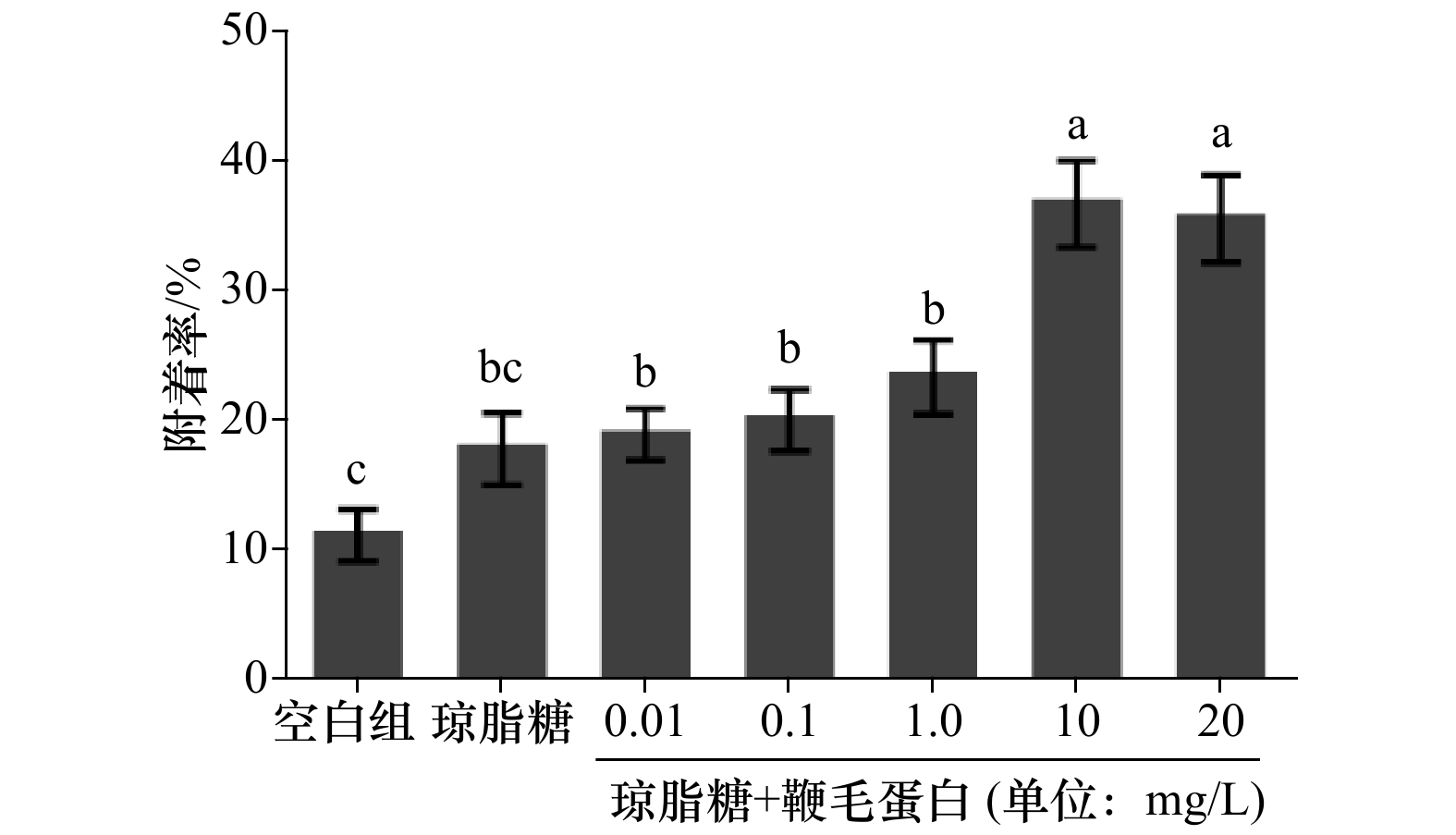
图 3 不同处理方式形成的生物被膜对稚贝附着的影响及细菌密度的分析
A. 鞭毛蛋白用不同方式处理P. marina细菌生物被膜后对厚壳贻贝稚贝的诱导作用; B. 鞭毛蛋白对P. marina 生物被膜上细菌密度的影响
Fig. 3 Influence of biofilms formed by different treatments on the settlement of plantigrades and the analysis of bacterial density
A. Percentages of settlement M. coruscus plantigrades on P. marina biofilm by flagellin is treated in different ways; B. effect of flagellin on bacterial density of P. marina biofilm
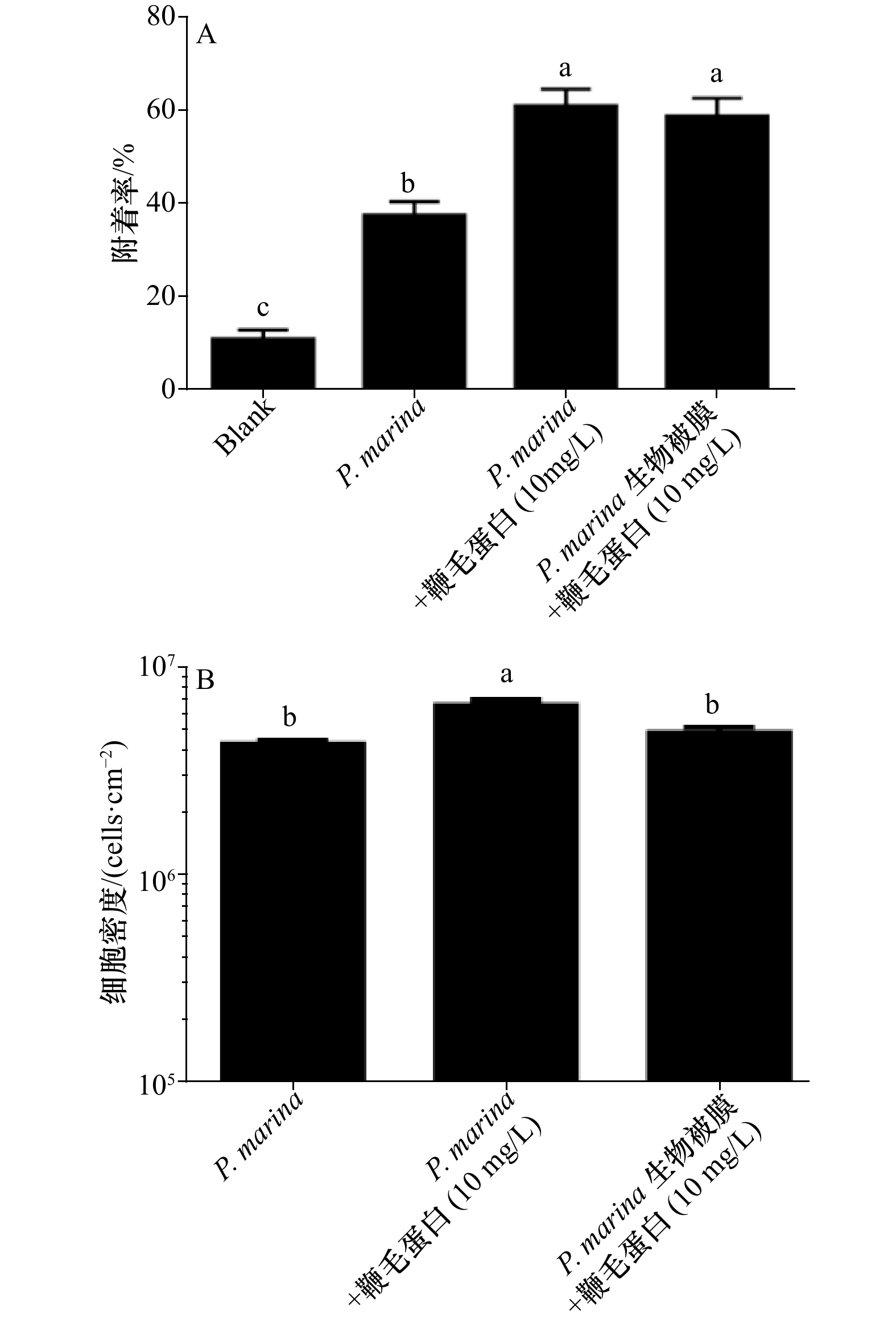
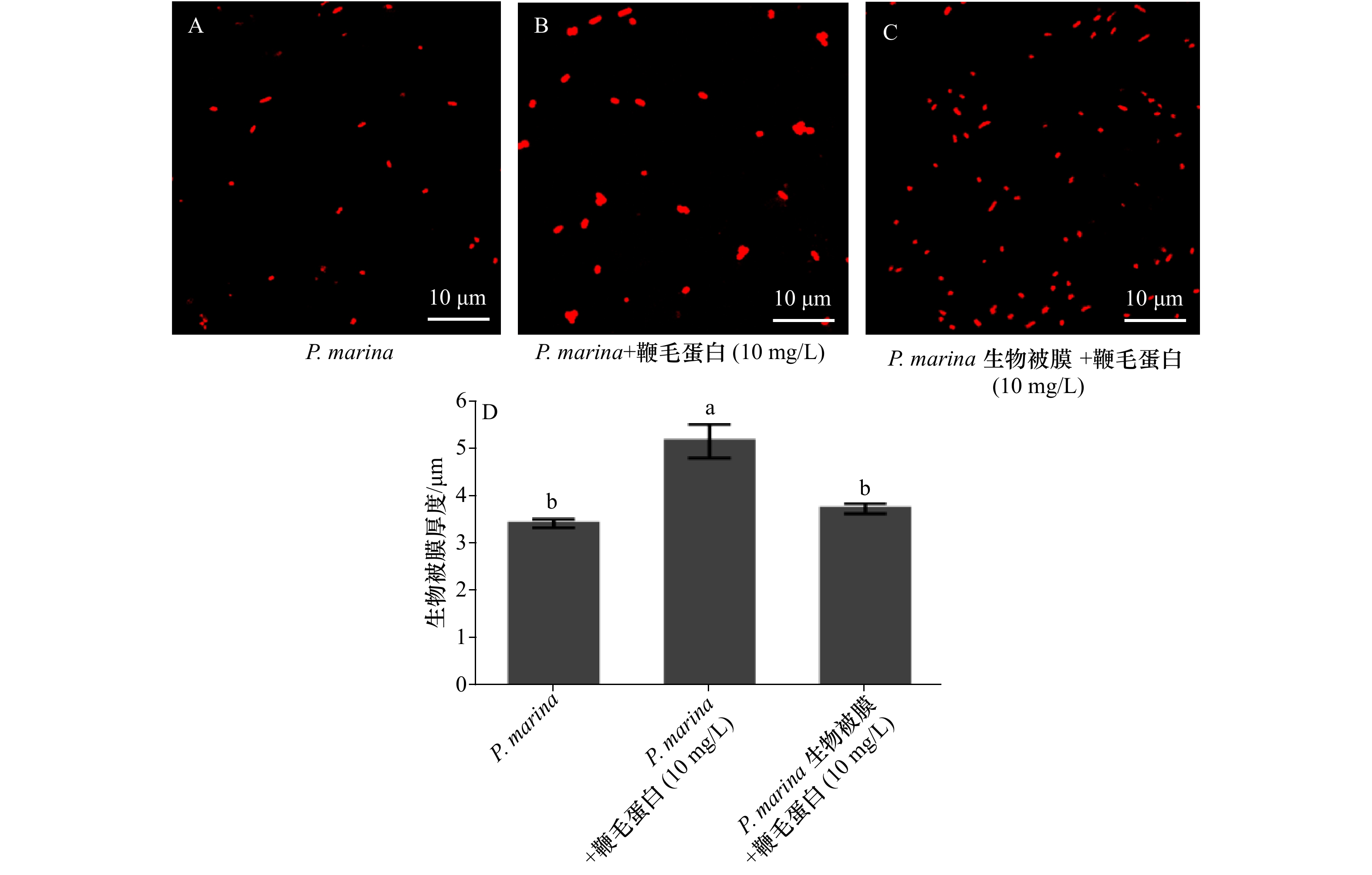
图 4 激光共聚焦扫描显微镜下鞭毛蛋白对P. marina 生物被膜上细菌分布与聚集状态的影响以及鞭毛蛋白对P. marina生物被膜膜厚膜厚的影响
A. 单一生物被膜上细菌密度;B. P. marina与鞭毛蛋白共同形成被膜上的细菌密度;C. P. marina生物被膜添加鞭毛蛋白后膜上细菌密度; D. 经鞭毛蛋白处理后的生物被膜膜厚分析
Fig. 4 Effect of flagellin on the distribution and aggregation of bacteria on P. marina biofilm by laser confocal scanning microscopy and the effect of flagellin on thickness of P. marina biofilm
A. Bacterial density on a single biofilm; B. the bacterial density on biofilm formed by P. marina and flagellin; C. the bacterial density on P. marina biofilm with flagellin; D. analysis of biofilm thickness after flagellin treatment
图 5 胞外产物含量
A. 鞭毛蛋白对P. marina 生物被膜上α-多糖、β-多糖、蛋白和脂类含量的影响;B. 鞭毛蛋白对P. marina 生物被膜上α-多糖、β-多糖、蛋白和脂类含量的统计及差异
Fig. 5 The extracellular polymeric substance
A. Effects of flagellin on the contents of α-polysaccharides, β-polysaccharides, proteins and lipids of P. marina biofilm; B. statistics and difference of α-polysaccharides, β-polysaccharides, proteins and lipids contents on P. marina biofilm by flagellin protein
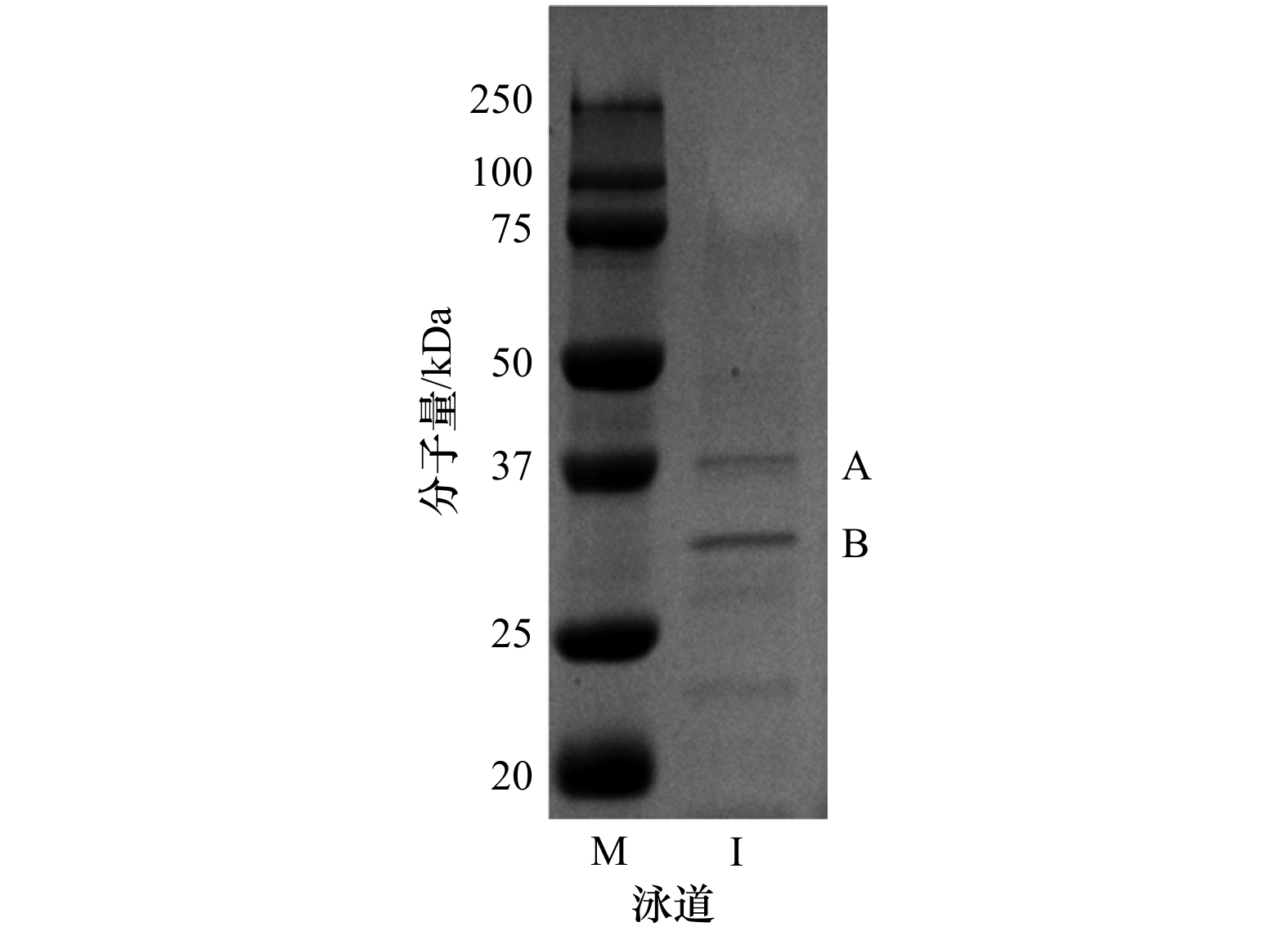
表 1 P. marina细菌鞭毛蛋白质谱分析鉴定结果
Tab. 1 The mass spectrometry identification of flagellin extracted from P. marina
蛋白条带 蛋白名称 相似度/% 多肽/Da 独特性/% 分子量/Da A 4-fliC 61 30 14 33 469 2-fliC 56 27 15 33 464 3-fliC 55 21 12 33 442 B 2-fliC 42 15 7 33 464 4-fliC 42 14 4 33 469 3-fliC 39 13 8 33 442 -
[1] 常亚青. 贝类增养殖学[M]. 北京: 中国农业出版社, 2007.Chang Yaqing. Mollusc Culture[M]. Beijing: China Agriculture Press, 2007. [2] 李太武. 海洋生物学[M]. 北京: 海洋出版社, 2013.Li Taiwu. Marine Biology[M]. Beijing: China Ocean Press, 2013. [3] 周轩, 郭行磐, 陈芋如, 等. 低湿度表面的海洋附着细菌对厚壳贻贝附着的影响[J]. 大连海洋大学学报, 2015, 30(1): 30−35. doi: 10.3969/J.ISSN.2095-1388.2015.01.006Zhou Xuan, Guo Xingpan, Chen Yuru, et al. Effects of bacterial biofilms formed on low surface wettability on settlement of plantigrades of the mussel Mytilus coruscus[J]. Journal of Dalian Ocean University, 2015, 30(1): 30−35. doi: 10.3969/J.ISSN.2095-1388.2015.01.006 [4] 张朝霞, 柯才焕, 冯丹青, 等. 海洋附着细菌对冠瘤海鞘幼体附着和变态的影响[J]. 海洋学报, 2005, 27(5): 96−102.Zhang Zhaoxiao, Ke Caihuan, Feng Danqing, et al. Influences of marine adhesive bacteria on settlement and metamorphosis of Styela conopus Savigny larvae[J]. Haiyang Xuebao, 2005, 27(5): 96−102. [5] Yang Jinlong, Shen Peijing, Liang Xiao, et al. Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to monospecific bacterial biofilms[J]. Biofouling, 2013, 29(3): 247−259. doi: 10.1080/08927014.2013.764412 [6] Yang Jinlong, Li Xiang, Liang Xiao, et al. Effects of natural biofilms on settlement of plantigrades of the mussel Mytilus coruscus[J]. Aquaculture, 2014, 424−425: 228−233. doi: 10.1016/j.aquaculture.2014.01.007 [7] Zeng Zhenshun, Guo Xingpan, Li Baiyuan, et al. Characterization of self-generated variants in Pseudoalteromonas lipolytica biofilm with increased antifouling activities[J]. Applied Microbiology and Biotechnology, 2015, 99(23): 10127−10139. doi: 10.1007/s00253-015-6865-x [8] Zeng Zhenshun, Guo Xingpan, Cai Xingsheng, et al. Pyomelanin from Pseudoalteromonas lipolytica reduces biofouling[J]. Microbial Biotechnology, 2017, 10(6): 1718−1731. doi: 10.1111/1751-7915.12773 [9] Roy K, Hilliard G M, Hamilton D J, et al. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells[J]. Nature, 2009, 457(7229): 594−598. doi: 10.1038/nature07568 [10] Liang Xiao, Zhang Xiukun, Peng Lihua, et al. The flagellar gene regulates biofilm formation and mussel larval settlement and metamorphosis[J]. International Journal of Molecular Sciences, 2020, 21(3): 710. doi: 10.3390/ijms21030710 [11] Ibrahim G F, Fleet G H, Lyons M J, et al. Method for the isolation of highly purified Salmonella flagellins[J]. Journal of Clinical Microbiology, 1985, 22(6): 1040−1044. doi: 10.1128/JCM.22.6.1040-1044.1985 [12] Li Zaiping. Molecular cloning: a laboratory manual (3rd Edition)[J]. Science Bulletin, 2002, 47(24): 1888. [13] Peng Lihua, Liang Xiao, Guo Xingpan, et al. Complete genome of Pseudoalteromonas marina ECSMB14103, a mussel settlement-inducing bacterium isolated from the East China Sea[J]. Marine Genomics, 2018, 41: 46−49. doi: 10.1016/j.margen.2018.04.001 [14] 杨金龙, 郭行磐, 陈芋如, 等. 中湿度表面的海洋细菌对厚壳贻贝稚贝附着的影响[J]. 水产学报, 2015, 39(3): 421−428.Yang Jinlong, Guo Xingpan, Chen Yuru, et al. Effects of bacterial biofilms formed on middle wettability surfaces on settlement of plantigrades of the mussel Mytilus coruscus[J]. Journal of Fisheries of China, 2015, 39(3): 421−428. [15] González-Machado C, Capita R, Riesco-Peláez F, et al. Visualization and quantification of the cellular and extracellular components of Salmonella Agona biofilms at different stages of development[J]. PLoS One, 2018, 13(7): e0200011. doi: 10.1371/journal.pone.0200011 [16] Maki J S, Rittschof D, Costlow J D, et al. Inhibition of attachment of larval barnacles, Balanus amphitrite, by bacterial surface films[J]. Marine Biology, 1988, 97(2): 199−206. doi: 10.1007/BF00391303 [17] 颜成英, 汤晓艳, 王敏, 等. 鼠伤寒沙门氏菌鞭毛蛋白提取及多抗制备[J]. 食品工业科技, 2014, 35(22): 176−178, 183.Yan Chengying, Tang Xiaoyan, Wang Min, et al. Extraction of flagellin from Salmonella typhimurium and preparation of its polyclonal antibody[J]. Science and Technology of Food Industry, 2014, 35(22): 176−178, 183. [18] Aschtgen M S, Brennan C A, Nikolakakis K, et al. Insights into flagellar function and mechanism from the squid-vibrio symbiosis[J]. NPJ Biofilms and Microbiomes, 2019, 5: 32. doi: 10.1038/s41522-019-0106-5 [19] Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili[J]. Molecular Microbiology, 1998, 30(2): 285−293. doi: 10.1046/j.1365-2958.1998.01061.x [20] Kim T J, Young B M, Young G M. Effect of flagellar mutations on Yersinia enterocolitica biofilm formation[J]. Applied and Environmental Microbiology, 2008, 74(17): 5466−5474. doi: 10.1128/AEM.00222-08 [21] Römling U, Galperin M Y. Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions[J]. Trends in Microbiology, 2015, 23(9): 545−557. doi: 10.1016/j.tim.2015.05.005 [22] Thongsomboon W, Serra D O, Possling A, et al. Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose[J]. Science, 2018, 359(6373): 334−338. doi: 10.1126/science.aao4096 [23] 梁箫, 童欢, 彭莉华, 等. 纤维素对海洋细菌生物被膜形成及厚壳贻贝幼虫附着变态的调控[J]. 大连海洋大学学报, 2020, 35(1): 75−82.Liang Xiao, Tong Huan, Peng Lihua, et al. Regulation of formation of biofilms and larval settlement and metamorphosis of musselMytilus coruscus by cellulose[J]. Journal of Dalian Ocean University, 2020, 35(1): 75−82. -




 下载:
下载: