Algal light-harvesting system: Linkage of structures and functions by using structural biology
-
摘要: 藻类是光合自养的水生孢子植物,为了适应水下弱光的特殊生境,藻类捕光天线历经亿万年的进化,形成了特殊的结构与功能。从发现藻类捕光天线的存在到至今的70多年间,其结构解析技术的发展共经历了4个阶段:首先是利用生化及普通光谱技术研究结构组成(1950−1980年);其次是利用X-ray晶体学技术研究局部精细结构(1980年至今);再次是利用电镜技术研究完整的粗略结构(1980−2010年);最后是近10年来利用冷冻电镜技术研究完整的精细结构(2010年至今)。目前以蓝藻、红藻、绿藻和硅藻为主的藻类捕光天线复合体完整的精细结构均已被解析,仅2019年就有10余种精细结构被发现。藻类捕光天线系统结构生物学的研究,不仅搭建了对结构与功能统一认识的桥梁,而且为深入揭示藻类光合作用高效能量传递机制奠定了坚实的结构基础。将藻类捕光天线系统结构和功能统一起来,进一步研究对光环境的适应性成为未来的重点,并将为藻类捕光天线蛋白在光电器件领域的应用提供充分的科学依据。Abstract: Algae are general term of a large group of photoautotrophic aquatic sporophytes. Along with the long earth history, the algal light-harvesting antenna has evolved special structure and function, to adapt to low-light underwater environment. Since the algal light-harvesting antennas were first discovered 70 years ago, the progress of structural analysis can be divided into four stages. The first stage was from 1950 to 1980, and effects were focused on studying the structural composition of light-harvesting antenna through biochemical and spectral techniques. The second stage was from 1980 to the present, and X-ray crystallization becomes a primary tool to study the partial fine-structure of the complete complex. The third stage was from 1980 to 2010. In this stage, complete contour structure can be studied by using electron microscope (EM). The fourth stage is from 2010 to the present, and the use of cryo-EM technology to studied intact fine-structure has brought the blowout period of structural analysis in recent year. With the rapid development of cryo-EM technology, a variety of complete fine-structures of algal light-harvesting antenna complexes have been analyzed, including cyanobacteria, red algae, green algae, and diatoms. Specifically, in 2019, multiple super-molecular complex structures of algal light-harvesting antenna were resolved. This breakthrough provides us valuable structure information for the study of energy transfer and the unified relationship between structure and function. However, the synthetic understanding of the relationship between the structure, function, environment, and applications of algal light-harvesting antennas is still preliminary, compared to the huge demand of solar energy utilization from bio-materials. Therefore, further research on the light adaptability of light-harvesting proteins has become the focus of the future, and will provide a sufficient scientific basis for the application of algal light-harvesting antenna proteins in the field of photoelectric devices.
-
图 1 捕光复合体粗略结构及精细结构对比
A. 红藻(Porphyridium cruentum)PBS 电镜结构(粗略结构)(图片引自文献[24]);B. 红藻(P. purpureum)PBS 冷冻电镜精细结构(图片源于PDB库,PDB码为6KGX);C. 豌豆C2S2M2-type PSII-LHCII 电镜结构(粗略结构)(图片引自文献[79]);D. 绿藻(Chlamydomonas reinhardtii)C2S2M2N2-type PSII-LHCII 冷冻电镜精细结构(图片源于PDB库,PDB码为6KAF)
Fig. 1 Comparison of contour and fine structures of algal light-harvesting complexes
A. The rough structure of PBS from red alga Porphyridium cruentum by using electron microscope (picture quoted from reference [24]); B. the fine-structure of PBS from red alga P. purpureum by using cryo-electron microscope (PDB code is 6KGX); C. the electron microscope structure (rough structure) of C2S2M2-type PSII-LHCII from pea (picture quoted from reference [79]); D. the fine cryo-electron microscope structure of C2S2M2N2-type PSII-LHCII from Chlamydomonas reinhardtii (picture quoted from PDB library, PDB code is 6KAF)
图 2 计算生物学分析蓝藻藻胆蛋白同源序列及结构[90]
A为通过计算生物学比对蓝藻的藻胆蛋白同源序列,识别非同义替换升高的位点(后验概率大于80%);横轴为藻胆蛋白中的氨基酸排列位点;纵轴为后验概率;A、B、E、F、F’、G、H代表7个螺旋,X、Y代表每个亚基N段的螺旋发卡域;浅灰色为α亚基,暗灰色为β亚基;图内部的a−d分支分别为PEC、PC、PE和APC。B为蓝藻藻蓝蛋白三维结构;灰色球状填充为dN/dS(同义频率/非同义频率)替换比例升高的残基;螺旋状条带表示蛋白质的α亚基和β亚基;灰色杆状表示生色团
Fig. 2 Computational biology analysis of cyanobacterial phycobiliprotein homologous sequence and structure [90]
A represent alignment of cyanobacterial phycobiliprotein homologous sequences to identify sites with increased non-synonymous substitutions (posterior probabilities>80%); the horizontal axis is amino acid arrangement sites; the vertical axis is posterior probability; A, B, E, F, F’, G, and H represent seven helices, X and Y represent the spiral hairpin domain at the N-terminus of each subunit; the gray lines represent α subunit, the dark gray lines represent β subunit; a-d represent PEC, PC, PE and APC, respectively. B represent three-dimensional structure of cyanobacterial phycocyanin; gray spheres represent the residues with increased synonymous frequency/non-synonymous frequency substitution ratio; helixes represent α subunit and β subunit; gray lines represent chromophore
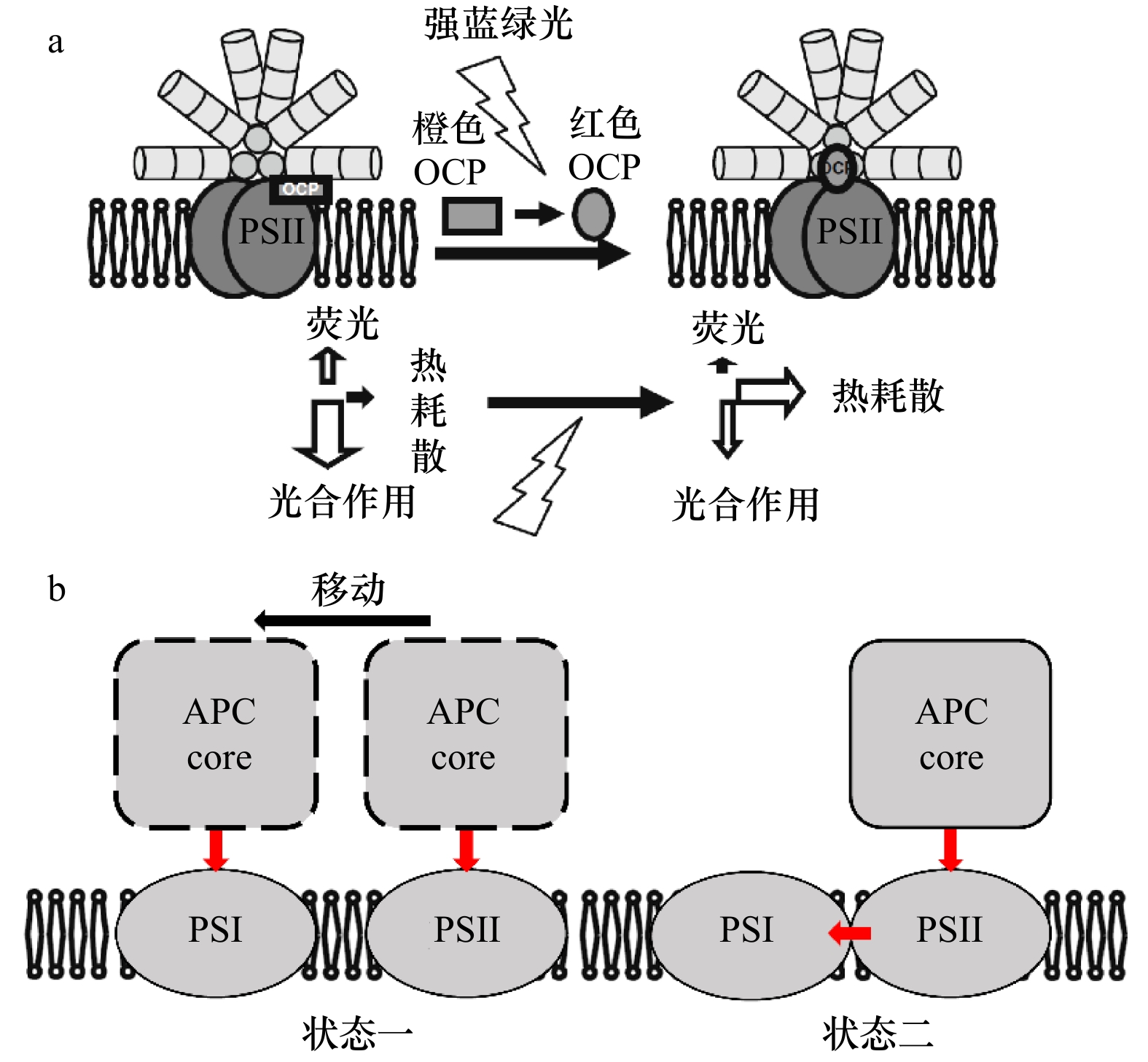
图 4 藻胆体光保护结构模型
a. 藻胆体的OCP依赖型NPQ机制模型,图片引自文献[103];b. 藻胆体状态转换模型,状态一为移动传能机制,状态二为能量溢出机制,红色箭头为能量传递路径
Fig. 4 Photoprotective structural model of phycobilisomes
a. The mechanism model of OCP-dependent NPQ in phycobilisomes, picture quoted from reference [103]; b. phycobilisome state transition model, state 1 represents mobile energy transfer mechanism, state 2 represents overflow mechanism, red arrow represents energy transfer path
表 1 藻类捕光天线复合体粗略结构及精细结构
Tab. 1 Contour model and fine structures of algal light-harvesting antenna complexes
捕光天线类型 粗略结构
鉴定方法结构结果 与功能的关系 引用文献 精细结构
鉴定方法结构结果 与功能的关系 引用文献 PBS 生理生化+
光谱技术蛋白凝胶电泳鉴定藻胆体由藻胆蛋白及各种连接多肽组成;光谱测量藻胆体光吸收范围为460~700 nm 
揭示捕光范围及可能的
能量传递路径[29] X-ray 藻胆蛋白脱辅基蛋白结构、结合色素类型及色素结合位点 
通过结合色素类型
及结合方式预测
能量传递路径[41] 普通电镜 PBS结构具有多样性,如半椭圆形、块状等 结合藻类生境,对比多种PBS形状及
粗略结构,可揭示PBS形状与周围
光环境的适应规律[21] 冷冻电镜 PBS完整结构及细节结构,如各类连接蛋白组成的骨架形式;色素网络及色素结合位点 揭示PBS组装机制及
能量传递途径[42] LHC及其与光系统的复合物 生理生化+
光谱技术蛋白凝胶电泳鉴定LHC蛋白大小及部分超分子复合体含有LHC的数量;光谱测量LHC光吸收范围为350~700 nm 

揭示捕光范围 [43] X-ray 脱辅基蛋白结构、结合色素类型及色素结合位点 
揭示内部能量
传递路径[36] 普通电镜 超分子复合体可能的组成成分,例如观察不同藻类的PSII-LHCII复合体中LHC的排列方式及数量 通过LHC与光合反应中心的结合
方式推测可能的能量传递方式[44] 冷冻电镜 复合体完整精细结构 能量传递途径及
光保护机制[45] 注:图片1引自文献[29]。图片1:SDS-聚丙烯酰胺凝胶电泳-Synechococcus PCC 6701的PBS多肽。图片2:隐藻(Chroomonas sp. CCMP270)捕光天线PC645晶体结构,结构下载于PDB(http://www.rcsb.org/),PDB码:4LMS,分辨率为1.35 Å。图片3和图片4引自文献[43]。图片3:褐藻Fx and Chl a/c bindingprotein A (FCPA)提取图;A:Triton-X-100样品提取处理;B:蔗糖梯度(0.2~0.5 mol/L蔗糖)样品分离。图片4:褐藻FCPA吸收光谱(波长450 nm处的上方线)及荧光发射光谱(波长450 nm处的下方线)的对比图。图片5:硅藻捕光天线FCP晶体结构,结构下载于PDB(http://www.rcsb.org/),PDB码为6A2W,分辨率为1.8 Å。 表 2 藻类捕光天线复合体晶体结构解析进展
Tab. 2 Progress in crystal structure analysis of algal light-harvesting antenna complexes
藻类 PSII主要捕光天线 PSI主要捕光天线 结合色素 文献 蓝藻 PBS: APC*, PC*, PE*, PEC (phycoerythrocyanin)* IsiA (Iron-stress-induced protein) PCB, PEB, PUB, PVB, Chl a [26, 46-48] 红藻 PBS: APC*, PC*, PE* LHCR: Lhcr PCB, PEB, PUB, Chl a [5, 21, 49-51] 绿藻 LHCII: Lhcb1-3, CP24, CP26, CP29* LHCI*: Lhca Chl a, Chl b [52-54] 隐藻 Cr(Cryptophytes)-PBP (phycobiliprotein): Cr-PC*/Cr-PE*; LHC: Lhcr, Lhcz 同PSII PCB, PEB, MBV(mesobiliverdin), DBV(dihydrobiliverdin), bilin 584, bilin 618, Chl a, Chl c, alloxanthin [55] 硅藻 FCP*: Lhcf, Lhcx 同PSII Chl a, Chl c, Fx, Dt, Dd [56-58] 褐藻 FCP: Lhcf, Lhcr, Lhcx, Lhcz 同PSII Chl a, Chl c, Fx,violaxanthin, zeaxanthin, Dt, Dd [43, 58-59] 甲藻 PCP*;LHC: Lhcr, Lhcf, Lhcx 同PSII Chl a, Chl c, Fx, violaxanthin [60-61] 黄藻 XLH (Xanthophyceae light-harvesting complex) II XLHI Chl a, Chl c, Dd, Dt [62] 金藻 LHC LHC Chl a, Chl c, Fx, violaxanthin [63-64] 裸藻 LHC LHC Chl a, Chl b, neoxanthin, Dt, Dd, β-carotene [65-67] 轮藻 LHC LHC Chl a, Chl b, α-carotene, β-carotene, γ-carotene, lutein, eaxanthin, neoxanthin, zeaxanthin, violaxanthin, [65-67] 注:*代表已有高分辨晶体结构。 表 3 电镜技术解析藻类捕光复合体结构的研究进展
Tab. 3 Advance in electron microscope analysis of algal light-harvesting complexes
藻类 捕光天线复合体 电镜 冷冻电镜 解析分辨率(冷冻电镜) 引用文献 蓝藻 IsiA-PSI √ √ 3.5 Å [76, 80] PBS √ 13 Å [25] PBSrod-PSI √ − [81] PBS-PSII √ − [82-83] 红藻 PSI-LHCI √ 3.82 Å [74] PBS √ 3.5 Å, 2.82 Å [22, 42] 绿藻 PSI-LHCI √ 3.49 Å, 2.9 Å [75, 84] PSII-LHCII √ 3.2 Å [85] C2S2-type PSII-LHCII √ 2.7 Å [45] C2S2M2-type PSII-LHCII √ 3.2 Å [86] C2S2M2N2-type PSII LHCII √ 3.7 Å [74] C2S2M2L2-type PSII LHCII √ 3.4 Å [45] PSI-LHCI-LHCII √ − [44] 硅藻 PSI-FCPI √ √ − [87-88] PSII-FCPII √ 3.02 Å, 3.8 Å [78, 89] 隐藻 PSI-LHCI √ − [72] 黄藻 XLH √ − [71] 注:*代表已有高分辨晶体结构,√代表已用该技术进行了结构解析,−代表无分辨率报道。 -
[1] McFadden G I. Primary and secondary endosymbiosis and the origin of plastids[J]. Journal of Phycology, 2001, 37(6): 951−959. doi: 10.1046/j.1529-8817.2001.01126.x [2] McFadden G I. Endosymbiosis and evolution of the plant cell[J]. Current Opinion in Plant Biology, 1999, 2(6): 513−519. doi: 10.1016/S1369-5266(99)00025-4 [3] 王寅初, 秦松. 真核藻类进化的研究进展与存在问题[J]. 生物学杂志, 2015, 32(3): 70−72. doi: 10.3969/j.issn.2095-1736.2015.03.070Wang Yinchu, Qin Song. Evolution of eukaryotic algae: a mini-review of progress and problems[J]. Journal of Biology, 2015, 32(3): 70−72. doi: 10.3969/j.issn.2095-1736.2015.03.070 [4] Seckbach J. Enigmatic Microorganisms and Life in Extreme Environments[M]. Netherlands: Springer, 1999: 5775. [5] Neilson J A D, Durnford D G. Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes[J]. Photosynthesis Research, 2010, 106(1/2): 57−71. [6] Glazer A N. Light guides: Directional energy transfer in a photosynthetic antenna[J]. The Journal of Biological Chemistry, 1989, 264(1): 1−4. [7] Krawczyk S, Krupa Z, Maksymiec W. Stark spectra of chlorophylls and carotenoids in antenna pigment-proteins LHC-II and CP-II[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1993, 1143(3): 273−281. doi: 10.1016/0005-2728(93)90198-O [8] Büchel C. Fucoxanthin-chlorophyll-proteins and non-photochemical fluorescence quenching of diatoms[M] //Demmig-Adams B, Garab G, Adams III W, et al. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Dordrecht: Springer, 2014: 259−275. [9] Mirkovic T, Ostroumov E E, Anna J M, et al. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms[J]. Chemical Reviews, 2017, 117(2): 249−293. doi: 10.1021/acs.chemrev.6b00002 [10] Onishi A, Aikawa S, Kondo A, et al. Energy transfer in Anabaena variabilis filaments under nitrogen depletion, studied by time-resolved fluorescence[J]. Photosynthesis Research, 2015, 125(1/2): 191−199. [11] Li Wenjun, Su Hainan, Pu Yang, et al. Phycobiliproteins: Molecular structure, production, applications, and prospects[J]. Biotechnology Advances, 2019, 37(2): 340−353. doi: 10.1016/j.biotechadv.2019.01.008 [12] Nelson N. Plant photosystem I—the most efficient nano-photochemical machine[J]. Journal of Nanoscience and Nanotechnology, 2009, 9(3): 1709−1713. doi: 10.1166/jnn.2009.SI01 [13] 马建飞, 林瀚智, 秦松, 等. 蓝藻藻胆体的体外组装研究进展[J]. 中国科学: 生命科学, 2016, 46(9): 1054−1061. doi: 10.1360/N052016-00067Ma Jianfei, Lin Hanzhi, Qin Song. Advances in cyanobacterial phycobilisome assembling in vitro[J]. Scientia Sinica Vitae, 2016, 46(9): 1054−1061. doi: 10.1360/N052016-00067 [14] Pu Yang, Zhu Guoliang, Ge Baosheng, et al. Photocurrent generation by recombinant allophycocyanin trimer multilayer on TiO2 electrode[J]. Chinese Chemical Letters, 2013, 24(2): 163−166. doi: 10.1016/j.cclet.2012.12.011 [15] Nagata M, Amano M, Joke T, et al. Immobilization and photocurrent activity of a light-harvesting antenna complex II, LHCII, isolated from a plant on electrodes[J]. ACS Macro Letters, 2012, 1(2): 296−299. doi: 10.1021/mz200163e [16] 马建飞. 藻胆体的冷冻电镜三维重构及应用——以海洋红藻Griffithsia pacifica和嗜热蓝薄Thermosynechococcus vulcanus藻胆体为例[D]. 烟台: 中国科学院烟台海岸带研究所, 2015.Ma Jianfei. 3-D reconstructions of phycobilisomes by cryo-electron microscopy and application—taking examples of phycobilisomes from the marine red alga Griffithsia pacifica and the thermophilic cyanobacterium Thermosynechococcus vulcanus[D]. Yantai: Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, 2015. [17] Ma Jianfei, Chen Huaxin, Qin Song, et al. Applications of natural and artificial Phycobiliproteins in solar cells[J]. Current Biotechnology, 2015, 4(3): 275−281. doi: 10.2174/2211550104666151016203528 [18] Thornber J P, Sokoloff M K. Photochemical reactions of purple bacteria as revealed by studies of three spectrally different carotenobacteriochlorophyll-protein complexes isolated from Chromatium, strain D[J]. Biochemistry, 1970, 9(13): 2688−2698. doi: 10.1021/bi00815a017 [19] Jones R F, Blinks L R. The amino acid constituents of the phycobilin chromoproteins of the red alga Porphyra[J]. The Biological Bulletin, 1957, 112(3): 363−370. doi: 10.2307/1539129 [20] Haxo F, O'hEocha C, Norris P. Comparative studies of chromatographically separated phycoerythrins and phycocyanins[J]. Archives of Biochemistry and Biophysics, 1955, 54(1): 162−173. doi: 10.1016/0003-9861(55)90019-9 [21] Gantt E, Lipschultz C A. Phycobilisomes of Porphyridium cruentum. I. Isolation[J]. The Journal of Cell Biology, 1972, 54(2): 313−324. doi: 10.1083/jcb.54.2.313 [22] Zhang Jun, Ma Jianfei, Liu Desheng, et al. Structure of phycobilisome from the red alga Griffithsia pacifica[J]. Nature, 2017, 551(7678): 57−63. doi: 10.1038/nature24278 [23] Arteni A A, Ajlani G, Boekema E J. Structural organisation of phycobilisomes from Synechocystis sp. strain PCC6803 and their interaction with the membrane[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2009, 1787(4): 272−279. doi: 10.1016/j.bbabio.2009.01.009 [24] Arteni A A, Liu Luning, Aartsma T J, et al. Structure and organization of phycobilisomes on membranes of the red alga Porphyridium cruentum[J]. Photosynthesis Research, 2008, 95(2/3): 169−174. doi: 10.1007/s11120-007-9264-z [25] Yi Zhiwei, Huang Hui, Kuang Tingyun, et al. Three-dimensional architecture of phycobilisomes from Nostoc flagelliforme revealed by single particle electron microscopy[J]. FEBS Letters, 2005, 579(17): 3569−3573. doi: 10.1016/j.febslet.2005.05.033 [26] Gingrich J C, Lundell D J, Glazer A N. Core substructure in cyanobacterial phycobilisomes[J]. Journal of Cellular Biochemistry, 1983, 22(1): 1−14. doi: 10.1002/jcb.240220102 [27] Myers J, Kratz W A. Relations between pigment content and photosynthetic characteristics in a blue-green alga[J]. The Journal of General Physiology, 1955, 39(1): 11−22. doi: 10.1085/jgp.39.1.11 [28] Yamanaka G, Glazer A N. Dynamic aspects of phycobilisome structure[J]. Archives of Microbiology, 1980, 124(1): 39−47. doi: 10.1007/BF00407026 [29] Glazer A N. Phycobilisomes[M]//Methods in Enzymology. Amsterdam: Elsevier, 1988: 304−312. [30] Robinson C V, Sali A, Baumeister W. The molecular sociology of the cell[J]. Nature, 2007, 450(7172): 973−982. doi: 10.1038/nature06523 [31] Hofmann E, Wrench P M, Sharples F P, et al. Structural basis of light harvesting by carotenoids: peridinin-chlorophyll-protein from Amphidinium carterae[J]. Science, 1996, 272(5269): 1788−1791. doi: 10.1126/science.272.5269.1788 [32] Camara-Artigas A, Bacarizo J, Andujar-Sanchez M, et al. PH-dependent structural conformations of B-phycoerythrin from Porphyridium cruentum[J]. The FEBS Journal, 2012, 279(19): 3680−3691. doi: 10.1111/j.1742-4658.2012.08730.x [33] David L, Marx A, Adir N. High-resolution crystal structures of trimeric and rod phycocyanin[J]. Journal of Molecular Biology, 2011, 405(1): 201−213. doi: 10.1016/j.jmb.2010.10.036 [34] Wilk K E, Harrop S J, Jankova L, et al. Evolution of a light-harvesting protein by addition of new subunits and rearrangement of conserved elements: crystal structure of a cryptophyte phycoerythrin at 1.63—A resolution[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(16): 8901−8906. doi: 10.1073/pnas.96.16.8901 [35] Doust A B, Marai C N J, Harrop S J, et al. Developing a structure-function model for the cryptophyte phycoerythrin 545 using ultrahigh resolution crystallography and ultrafast laser spectroscopy[J]. Journal of Molecular Biology, 2004, 344(1): 135−153. doi: 10.1016/j.jmb.2004.09.044 [36] Wang Wenda, Yu Longjiang, Xu Caizhe, et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms[J]. Science, 2019, 363(6427): eaav0365. doi: 10.1126/science.aav0365 [37] Liu Zhenfeng, Yan Hanchi, Wang Kebin, et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution[J]. Nature, 2004, 428(6980): 287−292. doi: 10.1038/nature02373 [38] Standfuss J, Van Scheltinga A C T, Lamborghini M, et al. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution[J]. The EMBO Journal, 2005, 24(5): 919−928. doi: 10.1038/sj.emboj.7600585 [39] Jordan P, Fromme P, Witt H T, et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution[J]. Nature, 2001, 411(6840): 909−917. doi: 10.1038/35082000 [40] Zouni A, Witt H T, Kern J, et al. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution[J]. Nature, 2001, 409(6821): 739−743. doi: 10.1038/35055589 [41] Harrop S J, Wilk K E, Dinshaw R, et al. Single-residue insertion switches the quaternary structure and exciton states of cryptophyte light-harvesting proteins[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(26): E2666−E2675. doi: 10.1073/pnas.1402538111 [42] Ma Jianfei, You Xin, Sun Shan, et al. Structural basis of energy transfer in Porphyridium purpureum phycobilisome[J]. Nature, 2020, 579(7797): 146−151. doi: 10.1038/s41586-020-2020-7 [43] Katoh T, Mimuro M, Takaichi S. Light-harvesting particles isolated from a brown alga, Dictyota dichotoma: a supramolecular assembly of fucoxanthin-chlorophyll-protein complexes[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1989, 976(2/3): 233−240. [44] Steinbeck J, Ross I L, Rothnagel R, et al. Structure of a PSI-LHCI-cyt b6f supercomplex in Chlamydomonas reinhardtii promoting cyclic electron flow under anaerobic conditions[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(41): 10517−10522. doi: 10.1073/pnas.1809973115 [45] Sheng Xin, Watanabe A, Li Anjie, et al. Structural insight into light harvesting for photosystem II in green algae[J]. Nature Plants, 2019, 5(12): 1320−1330. doi: 10.1038/s41477-019-0543-4 [46] Schmidt M, Krasselt A, Reuter W. Local protein flexibility as a prerequisite for reversible chromophore isomerization in α-phycoerythrocyanin[J]. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2006, 1764(1): 55−62. doi: 10.1016/j.bbapap.2005.10.022 [47] Andrizhiyevskaya E G, Schwabe T M E, Germano M, et al. Spectroscopic properties of PSI–IsiA supercomplexes from the cyanobacterium Synechococcus PCC 7942[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2002, 1556(2/3): 265−272. [48] Burnap R L, Troyan T, Sherman L A. The highly abundant chlorophyll-protein complex of iron-deficient Synechococcus sp. PCC7942 (CP43′) is encoded by the isiA gene[J]. Plant Physiology, 1993, 103(3): 893−902. doi: 10.1104/pp.103.3.893 [49] Ritter S, Hiller R G, Wrench P M, et al. Crystal structure of a phycourobilin-containing phycoerythrin at 1.90-Å resolution[J]. Journal of Structural Biology, 1999, 126(2): 86−97. doi: 10.1006/jsbi.1999.4106 [50] Gantt E. Pigment protein complexes and the concept of the photosynthetic unit: chlorophyll complexes and phycobilisomes[J]. Photosynthesis Research, 1996, 48(1/2): 47−53. [51] Wolfe G R, Cunningham F X, Durnfordt D, et al. Evidence for a common origin of chloroplasts with light-harvesting complexes of different pigmentation[J]. Nature, 1994, 367(6463): 566−568. doi: 10.1038/367566a0 [52] Bishop N I. The β, ϵ-carotenoid, lutein, is specifically required for the formation of the oligomeric forms of the light harvesting complex in the green alga, Scenedesmus obliquus[J]. Journal of Photochemistry and Photobiology B: Biology, 1996, 36(3): 279−283. doi: 10.1016/S1011-1344(96)07381-2 [53] Fawley M W, Stewart K D, Mattox K R. The novel light-harvesting pigment-protein complex of Mantoniella squamata (Chlorophyta): phylogenetic implications[J]. Journal of Molecular Evolution, 1986, 23(2): 168−176. doi: 10.1007/BF02099911 [54] Harrison M A, Melis A. Organization and stability of polypeptides associated with the chlorophyll a-b light-harvesting complex of photosystem-II[J]. Plant and Cell Physiology, 1992, 33(5): 627−637. [55] Bathke L, Rhiel E, Krumbein W E, et al. Biochemical and immunochemical investigations on the light-harvesting system of the cryptophyte rhodomonas sp. : evidence for a photosystem I specific antenna[J]. Plant Biology, 1999, 1(5): 516−523. doi: 10.1111/j.1438-8677.1999.tb00777.x [56] Gundermann K, Schmidt M, Weisheit W, et al. Identification of several sub-populations in the pool of light harvesting proteins in the pennate diatom Phaeodactylum tricornutum[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2013, 1827(3): 303−310. doi: 10.1016/j.bbabio.2012.10.017 [57] Eppard M, Rhiel E. The genes encoding light-harvesting subunits of Cyclotella cryptica (Bacillariophyceae) constitute a complex and heterogeneous family[J]. Molecular and General Genetics MGG, 1998, 260(4): 335−345. doi: 10.1007/s004380050902 [58] Caron L, Remy R, Berkaloff C. Polypeptide composition of light-harvesting complexes from some brown algae and diatoms[J]. FEBS Letters, 1988, 229(1): 11−15. doi: 10.1016/0014-5793(88)80787-7 [59] Cock J M, Sterck L, Rouzé P, et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae[J]. Nature, 2010, 465(7298): 617−621. doi: 10.1038/nature09016 [60] Durnford D G, Deane J A, Tan S, et al. A phylogenetic assessment of the eukaryotic light-harvesting antenna proteins, with implications for plastid evolution[J]. Journal of Molecular Evolution, 1999, 48(1): 59−68. doi: 10.1007/PL00006445 [61] Boldt L, Yellowlees D, Leggat W. Hyperdiversity of genes encoding integral light-harvesting proteins in the dinoflagellate Symbiodinium sp[J]. PLoS One, 2012, 7(10): e47456. doi: 10.1371/journal.pone.0047456 [62] Büchel C, Wilhelm C. Isolation and characterization of a photosystem I-associated antenna (LHC I) and a photosystem I—core complex from the chlorophyll c-containing alga Pleurochloris meiringensis (Xanthophyceae)[J]. Journal of Photochemistry and Photobiology B: Biology, 1993, 20(2/3): 87−93. [63] Lichtlé C, Arsalane W, Duval J C, et al. Characterization of the light-harvesting complex of Giraudyopsis stellifer (chrysophyceae) and effects of light stress[J]. Journal of Phycology, 1995, 31(3): 380−387. doi: 10.1111/j.0022-3646.1995.00380.x [64] Wiedemann I, Wilhelm C, Wild A. Isolation of chlorophyll-protein complexes and quantification of electron transport components in Synura petersenii and Tribonema aequale[J]. Photosynthesis Research, 1983, 4(1): 317−329. doi: 10.1007/BF00041829 [65] Simpson D J, Knoetzel J. Light-harvesting complexes of plants and algae: introduction, survey and nomenclature[M]//Ort D R, Yocum C F, Heichel I F. Oxygenic Photosynthesis: the Light Reactions. Dordrecht: Springer, 1996: 493−506. [66] Jeffrey S W. Algal pigment systems[M]//Falkowski P G. Primary Productivity in the Sea. Boston, MA: Springer, 1980: 33−58. [67] Schagerl M, Pichler C, Donabaum K. Patterns of major photosynthetic pigments in freshwater algae. 2. Dinophyta, Euglenophyta, Chlorophyceae and Charales[J]. Annales de Limnologie-International Journal of Limnology, 2003, 39(1): 49−62. doi: 10.1051/limn/2003005 [68] Wildman R B, Bowen C C. Phycobilisomes in blue-green algae[J]. Journal of Bacteriology, 1974, 117(2): 866−881. doi: 10.1128/JB.117.2.866-881.1974 [69] Zhang Yuzhong, Chen Xiulan, Zhou Baicheng, et al. A new model of phycobilisome in Spirulina platensis[J]. Science in China Series C: Life Sciences, 1999, 42(1): 74−79. doi: 10.1007/BF02881751 [70] Liu Luning, Aartsma T J, Thomas J C, et al. Watching the native supramolecular architecture of photosynthetic membrane in red algae: topography of phycobilisomes and their crowding, diverse distribution patterns[J]. The Journal of Biological Chemistry, 2008, 283(50): 34946−34953. doi: 10.1074/jbc.M805114200 [71] Gardian Z, Tichý J, Vácha F. Structure of PSI, PSII and antennae complexes from yellow-green alga Xanthonema debile[J]. Photosynthesis Research, 2011, 108(1): 25. doi: 10.1007/s11120-011-9647-z [72] Kereïche S, Kouřil R, Oostergetel G T, et al. Association of chlorophyll a/c2 complexes to photosystem I and photosystem II in the cryptophyte Rhodomonas CS24[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2008, 1777(9): 1122−1128. doi: 10.1016/j.bbabio.2008.04.045 [73] Bai Xiaochen, McMullan G, Scheres S H W. How cryo-EM is revolutionizing structural biology[J]. Trends in Biochemical Sciences, 2015, 40(1): 49−57. doi: 10.1016/j.tibs.2014.10.005 [74] Pi Xiong, Tian Lirong, Dai Huaien, et al. Unique organization of photosystem I-light-harvesting supercomplex revealed by cryo-EM from a red alga[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(17): 4423−4428. doi: 10.1073/pnas.1722482115 [75] Su Xiaodong, Ma Jun, Pan Xiaowei, et al. Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex[J]. Nature Plants, 2019, 5(3): 273−281. doi: 10.1038/s41477-019-0380-5 [76] Toporik H, Li Jin, Williams D, et al. The structure of the stress-induced photosystem I-IsiA antenna supercomplex[J]. Nature Structural & Molecular Biology, 2019, 26(6): 443−449. [77] Shen Liangliang, Huang Zihui, Chang Shenghai, et al. Structure of a C2S2M2N2-type PSII-LHCII supercomplex from the green alga Chlamydomonas reinhardtii[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(42): 21246−21255. doi: 10.1073/pnas.1912462116 [78] Nagao R, Kato K, Suzuki T, et al. Structural basis for energy harvesting and dissipation in a diatom PSII-FCPII supercomplex[J]. Nature Plants, 2019, 5(8): 890−901. doi: 10.1038/s41477-019-0477-x [79] Pagliano C, Nield J, Marsano F, et al. Proteomic characterization and three-dimensional electron microscopy study of PSII-LHCII supercomplexes from higher plants[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2014, 1837(9): 1454−1462. doi: 10.1016/j.bbabio.2013.11.004 [80] Bibby T S, Nield J, Barber J. Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria[J]. Nature, 2001, 412(6848): 743−745. doi: 10.1038/35089098 [81] Watanabe M, Semchonok D A, Webber-Birungi M T, et al. Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(7): 2512−2517. doi: 10.1073/pnas.1320599111 [82] Chang Leifu, Liu Xianwei, Li Yanbing, et al. Structural organization of an intact phycobilisome and its association with photosystem II[J]. Cell Research, 2015, 25(6): 726−737. doi: 10.1038/cr.2015.59 [83] Liu Haijun, Zhang Hao, Niedzwiedzki D M, et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria[J]. Science, 2013, 342(6162): 1104−1107. doi: 10.1126/science.1242321 [84] Qin Xiaochun, Pi Xiong, Wang Wenda, et al. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits[J]. Nature Plants, 2019, 5(3): 263−272. doi: 10.1038/s41477-019-0379-y [85] Wei Xuepeng, Su Xiaodong, Cao Peng, et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution[J]. Nature, 2016, 534(7605): 69−74. doi: 10.1038/nature18020 [86] Su Xiaodong, Ma Jun, Wei Xuepeng, et al. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex[J]. Science, 2017, 357(6353): 815−820. doi: 10.1126/science.aan0327 [87] Ikeda Y, Yamagishi A, Komura M, et al. Two types of fucoxanthin-chlorophyll-binding proteins I tightly bound to the photosystem I core complex in marine centric diatoms[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2013, 1827(4): 529−539. doi: 10.1016/j.bbabio.2013.02.003 [88] Nagao R, Ueno Y, Akita F, et al. Biochemical characterization of photosystem I complexes having different subunit compositions of fucoxanthin chlorophyll a/c-binding proteins in the diatom Chaetoceros gracilis[J]. Photosynthesis Research, 2019, 140(2): 141−149. doi: 10.1007/s11120-018-0576-y [89] Pi Xiong, Zhao Songhao, Wang Wenda, et al. The pigment-protein network of a diatom photosystem II-light-harvesting antenna supercomplex[J]. Science, 2019, 365(6452): eaax4406. doi: 10.1126/science.aax4406 [90] Zhao Fangqing, Qin Song. Evolutionary analysis of phycobiliproteins: implications for their structural and functional relationships[J]. Journal of molecular evolution, 2006, 63(3): 330−340. doi: 10.1007/s00239-005-0026-2 [91] Zhang J M, Shiu Y J, Hayashi M, et al. Investigations of ultrafast exciton dynamics in allophycocyanin trimer[J]. The Journal of Physical Chemistry A, 2001, 105(39): 8878−8891. doi: 10.1021/jp011266a [92] Fleming G R, Van Grondelle R. Femtosecond spectroscopy of photosynthetic light-harvesting systems[J]. Current Opinion in Structural Biology, 1997, 7(5): 738−748. doi: 10.1016/S0959-440X(97)80086-3 [93] Akimoto S, Yamazaki I, Murakami A, et al. Ultrafast excitation relaxation dynamics and energy transfer in the siphonaxanthin-containing green alga Codium fragile[J]. Chemical Physics Letters, 2004, 390(1/3): 45−49. [94] Hashimoto H, Sugisaki M, Yoshizawa M. Ultrafast time-resolved vibrational spectroscopies of carotenoids in photosynthesis[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2015, 1847(1): 69−78. doi: 10.1016/j.bbabio.2014.09.001 [95] Collini E, Wong C Y, Wilk K E, et al. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature[J]. Nature, 2010, 463(7281): 644−647. doi: 10.1038/nature08811 [96] Dean J C, Mirkovic T, Toa Z S D, et al. Vibronic enhancement of algae light harvesting[J]. Chem, 2016, 1(6): 858−872. doi: 10.1016/j.chempr.2016.11.002 [97] Goss R, Lepetit B. Biodiversity of NPQ[J]. Journal of Plant Physiology, 2015, 172: 13−32. doi: 10.1016/j.jplph.2014.03.004 [98] Pan Xiaowei, Ma Jun, Su Xiaodong, et al. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II[J]. Science, 2018, 360(6393): 1109−1113. doi: 10.1126/science.aat1156 [99] Olaya-Castro A, Scholes G D. Energy transfer from Förster-Dexter theory to quantum coherent light-harvesting[J]. International Reviews in Physical Chemistry, 2011, 30(1): 49−77. doi: 10.1080/0144235X.2010.537060 [100] 冷轩, 王专, 翁羽翔. 光合捕光天线系统的进化模式与能量传递[J]. 植物生理学报, 2016, 52(11): 1681−1691.Leng Xuan, Wang Zhuan, Weng Yuxiang. Evolution of photosynthetic light harvesting antenna systems and energy transfer efficiency[J]. Plant Physiology Journal, 2016, 52(11): 1681−1691. [101] Cao Jianshu, Cogdell R J, Coker D F, et al. Quantum biology revisited[J]. Science Advances, 2020, 6(14): eaaz4888. doi: 10.1126/sciadv.aaz4888 [102] Ramanan C, Ferretti M, Van Roon H, et al. Evidence for coherent mixing of excited and charge-transfer states in the major plant light-harvesting antenna, LHCII[J]. Physical Chemistry Chemical Physics, 2017, 19(34): 22877−22886. doi: 10.1039/C7CP03038J [103] Kirilovsky D. The photoactive orange carotenoid protein and photoprotection in cyanobacteria[M]//Hallenbeck P. Recent Advances in Phototrophic Prokaryotes. New York, NY: Springer, 2010: 139−159. [104] McConnell M D, Koop R, Vasil'ev S, et al. Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria: a structure-based model for the light state transition[J]. Plant Physiology, 2002, 130(3): 1201−1212. doi: 10.1104/pp.009845 [105] Adir N, Lerner N. The crystal structure of a novel unmethylated form of C-phycocyanin, a possible connector between cores and rods in pycobilisomes[J]. The Journal of Biological Chemistry, 2003, 278(28): 25926−25932. doi: 10.1074/jbc.M302838200 [106] Lüder U H, Knoetzel J, Wiencke C. Two forms of phycobilisomes in the Antarctic red macroalga Palmaria decipiens (Palmariales, Florideophyceae)[J]. Physiologia Plantarum, 2001, 112(4): 572−581. doi: 10.1034/j.1399-3054.2001.1120416.x -





 下载:
下载: